Gel Electrophoresis Objective
To perform SDS-PAGE Gel Electrophoresis for separation of proteins.
About Gel Electrophoresis
Almost most of the analytical electrophoresis of proteins is carried out in polyacrylamide gels under conditions (generally SDS-PAGE) that ensure dissociation of the proteins into their individual polypeptide subunits.
Most common anionic detergent SDS (Sodium Dodecyl Sulfate) is used in combination with a reducing agent such as 2-mercaptoethanol or dithiothreitol (DTT) followed by heating to dissociate or break ionic and disulphide bonds of the proteins before they are loaded on the gel for Gel Electrophoresis.
The denatured proteins or polypeptide binds SDS and become negatively charged.
Because the amount of SDS bound is proportional to the molecular weight of the polypeptide or protein and is independent of its sequence, SDS-polypeptide complexes migrate through polyacrylamide gels in accordance with the size of polypeptide.
Using various markers of known molecular weight, it is therefore possible to estimate the molecular weight of the polypeptide chain(s).
Hence, SDS-PAGE Gel Electrophoresis is the most widely used method for qualitatively analyzing any protein mixtures.
The method is based on the separation of proteins according to their size and then locating by binding them to a dye.
Gel Electrophoresis is particularly used for monitoring protein purity and to determine their relative mass.
Adopted from BioRender
In, most cases SDS-PAGE Gel Electrophoresis is carried out in a discontinuous buffer system in which buffer in the reservoirs is of a various pH gradient and ionic strength from the buffer used to cast the polyacrylamide gel.
The Sodium Dodecyl Sulfate (SDS)-polypeptide complexes in the protein sample that is applied to the gel are swept along by a moving fence created when an electric current is passed between the electrodes.
After moving through a stacking gel of high porosity, the protein complexes are stacked on the surface of the resolving gel.
The ability of discontinuous buffer systems is to concentrate all of the complexes into a very small volume greatly increases the resolution of Sodium Dodecyl Sulfate (SDS)-polyacrylamide gels.
Mechanism of Gel Polymerization in Electrophoresis
The sample and the stacking gel contain Tris-Cl (pH 6.8), and upper top and lower buffer tank contain Tris-glycine (pH 8.3), and the resolving gel contains Tris-Cl (pH 8.8).
All components of the system contain 0.1% SDS.
The gels are composed of chains of polymerized acrylamide that are cross-linked by a bifunctional agent such as N,N’ -methylene bisacrylamide. The effective range of separation of Sodium Dodecyl Sulfate (SDS)-polyacrylamide gels (SDS-PAGE ) depends on the concentration of polyacrylamide used to prepare the gel and on the amount of cross-linking.
Polymerization of acrylamide in absence of cross-linking agents generates viscous solutions that are of no practical use.
Cross-liks formed by bisacrylamide add rigidity and tensile strength to the gel and form pores through which the SDS-polypeptide complexes must pass.
The size of these pores decreases with the increase of bisacrylamide:acrylamide ratio, reaching a minimum when the ratio is approximately 1:20.
Most gels are cast with molar ratios of bisacrylamide:acrylamide of 1:29.
The sieving properties of the gel are evaluated by the size of the pore which is determined by the absolute concentrations of acrylamide and bisacrylamide.
The following table shows the linear range of separation obtained with gel cast with concentrations of acrylamide that range from 5% to 15%:
Acrylamide Concentration and Protein Size in Electrophoresis
| Acrylamide concentration (%) | Linear range of separation (kD) |
| 15 | 12-43 |
| 10 | 16-68 |
| 7.5 | 36-94 |
| 5.0 | 57-212 |
Component of Gel Electrophoresis
Separating / resolving gel:
The lower (separating, running or resolving) gel is prepared using 5-15% acrylamide which is more than that used in the stacking gel (the amount of acrylamide used depends upon the molecular weight of the macromolecule under separation).
Hence the pores are numerous and of a smaller diameter imparting the molecular sieving property to this gel.
It is in this gel that the macromolecules subsequently separate.
The separating gel constitutes about two-thirds of the length of the gel plates.
The buffer used in the running gel is Tris.Cl at pH 8.8.
Stacking gel:
The stacking gel is layered on top of the separating gel after it has polymerized completely.
It is prepared using 2-5% of acrylamide and is consequently highly porous and devoid of any molecular sieving action.
The stacking gel constitutes one-third of the length of the gel plates in which the comb is placed in order to form wells for sample loading.
The buffer used in preparation of stacking gel is Tris.Cl. at pH 6.8.
Gel Electrophoresis Process
Glycine in the upper buffer reservoir exists in two forms; as a zwitterion which does not have a net charge, and as glycinate ion which is negatively charged.
When the power is switched on, chloride, protein and glycinate anions begin to migrate towards the anode.
Upon entering the stacking gel, the glycinate ions encounter a condition of low pH (pH of stacking gel buffer is about 2 pH units lower than that of the upper reservoir) which shifts the pH towards formation of zwitter ion.
Since zwitter ions are devoid of charge, they are immobile.
This immobility of glycine zwitterions to migrate into stacking gel coupled with high mobility of the chloride ions creates high localized voltage gradient between the leading chloride ion and the trailing glycinate ions.
Since protiens have their mobility intermediate between the trailing and the leading ions, the proteins carry the current in this region and migrate rapidly in this high local electric field.
However, they cannot overtake the chloride ions since the strong local fields exist only between the chloride and the glycinate ions.
As a consequence the proteins migrate quickly until they reach the chloride rich region and then drastically slow down.
This two speed movement of the proteins results in a piling up of protein sample in a tight sharp disc between the glycinate and the chloride ions.
It is in this form that the protein enters the resolving gel.
The small pore size of the gel retards the protein band for a while which allows the glycinate ions to catch up.
As soon as the glycinate ions enter the resolving gel they encounter a higher pH range and thus resume their full charge upon diasappearance of the localized high voltage gradient.
Now the separation of the proteins takes place according to their molecular weight.
Note: The buffer in the upper and the lower reservoir is Tris-glycine at pH 8.3, whereas the buffer used for preparing the stacking gel is Tris-Cl at pH 6.8.
Gel Electrophoresis Requirements
1. Acrylamide and N,N’ -methylene bisacrylamide: Prepare a stock solution containing 29% (w/v) acrylamide and 1% (w/v) bisacrylamide in deionized warm, water (to assist the dissolution of bisacrylamide).
Note: Acrylamide and bisacrylamide are slowly converted to acrylic and bisacrylic acid upon storage.
The reaction is catalyzed by light and alkali.
Hence always check that the pH of the solution is 7.0 or less, and store it in dark bottles at room temperature.
The fresh solutions should be prepared every few months.
Caution: Both are neurotoxins.
Polyacrylamide is considered to be non-toxic but care is to be taken while handling it as it might contain some amount of unpolymerized material.
2. Sodium dodecyl sulphate (SDS): Prepare a 10% (w/v) stock solution in Deionized water and store at room temperature.
3. Tris buffers for the preparation of resolving and stacking gels: Prepare 1.5 M Tris Cl. (pH 8.8) for resolving gel and 1.0 M Tris.Cl (pH 6.8) for stacking gel.
4. TEMED (N,N,N’N’-tetramethylenediamine): TEMED accelerates the polymerization of acrylamide and bisacrylamide by catalyzing the formation of free redicals from ammonium persulfate.
5. Ammonium persulfate (APS): It provides free radicals for polymerization of acrylamide and bisacrylamide.
Ammonium persulfate decomposes slowly and fresh solution is to be prepared weekly.
Prepare a 10% stock solution in Deionized water and store at 4C.
6. Tris-glycine electrophoresis buffer: 25 mM Tris base, 250 mM glycine and 0.1% SDS.
Adjust pH 8.3.
Prepare a 5X stock by dissolving 15.1 g of Tris base and 94 g of glycine in 900 ml water.
Adjust pH 8.3.
Add 50 ml of 10% (w/v) stock solution of SDS.
Make up the volume to 1000 ml with water.
7. 2X SDS gel-loading buffer: 100 mM Tris Cl (pH 6.8), 10% β-mercaptoethanol, 4% SDS, 0.2% bromophenol blue, 20% glycerol.
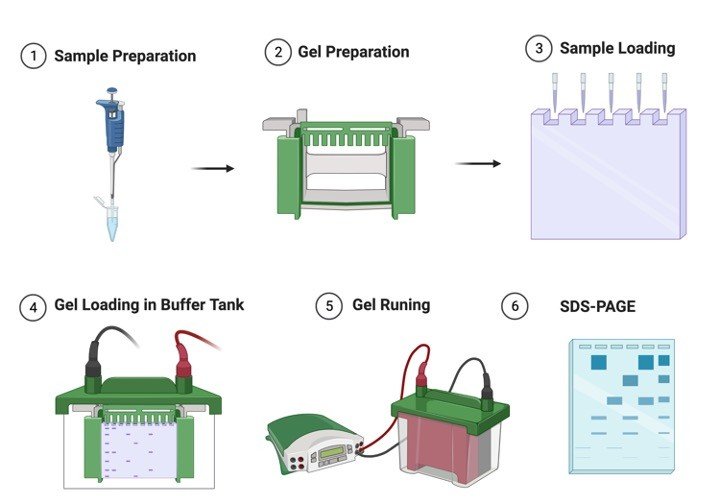
Gel Electrophoresis Procedure
1. Clean the whole Gel Electrophoresis apparatus with methanol so as to ensure them to be clean and detergent free.
Assemble the glass plates.
Clamp the assembly to the gel casting apparatus.
Ensure the Gel Electrophoresis assembly is leak proof by filling methanol between the plates where the gel is to be casted.
2. Determine the volume of the gel mold.
3. Prepare the resolving gel as shown below:
For preparing 15 ml, 12% gel
| Solution components | Volume in ml |
| Water | 4.9 |
| 30% acrylamide mix | 6.0 |
| 1.5M Tris.Cl (pH 8.8) | 3.8 |
| 10% SDS | 0.15 |
| 10% APS | 0.15 |
| TEMED | .006 |
Add APS and TEMED in the last because polymerization will begin as soon as TEMED has been added.
4. Pour 13 ml of acrylamide solution in the gap between the glass plates (leave sufficient space for the stacking gel- the length of the teeth of the comb plus 1 cm).
Carefully overlay the gel with isobutanol to prevent oxygen from diffusing into the gel (oxygen inhibits polymerization).
5. After polymerization is complete (around 30-40 min), pour off the isobutanol and wash the top of the gel several times with water so as to remove the traces of unpolymerized material.
Remove the remaining water using a filter paper towel.
6. Prepare the stacking gel as follows:
| Solution components | Volume in ml |
| Water | 5.5 |
| 30% acrylamide mix | 1.3 |
| 1.0M Tris.Cl (pH 6.8) | 1.0 |
| 10% SDS | 0.08 |
| 10% APS | 0.08 |
| TEMED | .008 |
8. Pour the stacking gel solution directly on to the surface of the polymerized resolving gel.
Immediately insert a clean Teflon comb (cleaned with water and dried with ethanol).
Avoid trapping air bubbles.
Add more stacking gel solution from the sides of the comb to fill the spaces completely.
9. After polymerization is complete (30-40 min), remove the Teflon comb, clean the wells with water to remove any unpolymerized material.
Mount the gel in the electrophoretic apparatus.
For ease mark the wells.
Add tris-glycine electrophoretic buffer to the top and the bottom reservoir.
First add the buffer in the upper reservoir and check for leak.
10. Load upto 20 ul of each sample in a pre-determined order into the bottom of the wells with a Hamilton microliter syringe.
Load the samples in an order so that the gel can be cut in two exact replicas after run so as to use one part for staining and other for blotting.
11. Attach the electrophoretic apparatus to electric power supply.
Positive electrode should be connected to the lower reservoir.
Apply voltage of 8V/cm to the gel.
When the dye front has moved into the resolving gel increase the voltage to 15V/cm and run the gel until the dye reaches the bottom of the gel.
12. Turn off the power supply. Remove the gel plates from the apparatus.
Carefully remove the gel with the help of spacers.
Cut it into two and place one part in a reservoir to stain with coomassie brilliant blue so as to visualize the protein bands, and use the other part for western blotting.
Store the part to be used for western blotting in transfer buffer (39 mM glycine, 48 mM Tris base, 0.037% SDS and 20% methanol, pH 8.3.
Make up the volume with water) at 4C.
Gel Staining
Polypeptides separated by SDS-PAGE Gel Electrophoresis can be simultaneously fixed with methanol:glacial acetic acid and stained with coomassie brilliant blue R250.
The gel is immersed for several hours in a concentrated methanol and acetic acid solution of the dye and the excess dye is then allowed to diffuse from the gel during a prolonged period of destaining.
1. Dissolve 0.25 g of coomassie brilliant blue R250 (COBBR-250) in 90 ml of methanol: H2O (1:1 v/v) and 10ml of glacial acetic acid.
Filter the solution through a whatmann No. 1 filter to remove any particulate matter.
2. Immerse the gel in atleast 5 volumes of staining solution and place on a slowly rocking platform for a minimum of 4 h at room temperature.
3. After that, remove the stain and save it for future use.
Destain the gel by soaking it in methanol/acetic acid solution (step 1) without the dye on a slowly rocking platform for 4-8 h, changing the destaining solution three or 4 times.
4. The more thoroughly the gel is destained, the smaller the amount of protein that can be detected by staining with COBBR250.
5. After destaining the gels can be stored indefinitely in water containing 20% glycerol in a sealed plastic bag.
Gel Electrophoresis Citations:
Share