Table of Contents
Enzyme Definition
A biomolecule that speeds up particular chemical processes by acting as a catalyst. Proteins and RNA (ribozymes) molecules are both examples of enzymes.
What is Enzyme?
An enzyme is a type of biomolecule that can be made biologically (naturally) or by other methods (synthetically). Its primary purpose is to act as a catalyst, speeding up a chemical reaction while remaining unchanged in the process. Enzymes are protein molecules with a particular amino acid sequence that folds to form a three-dimensional structure that provides the molecule with its unique characteristics.
Proteins are one of the main macromolecules, with carbohydrates (particularly polysaccharides), lipids, and nucleic acids rounding out the list. In nature, enzymes are proteins made up of polymers of amino acids. Peptide bonds connect the amino acids together. The DNA in the cell that generates the enzyme protein encodes the kind and sequence of amino acids in the enzyme protein. Proteins aren’t all enzymes, and enzymes aren’t all proteins.
Ribozymes are examples of enzymes that aren’t proteinaceous in nature. A ribozyme is an RNA-based enzyme rather than a protein-based enzyme. The ribosome, which is a combination of protein and catalytic RNA molecules, is an example of a ribozyme.
Enzyme Characteristics
Enzymes are frequently spherical in shape. They can be found alone or as part of a complex. They are frequently bigger than the substrates on which they grow. Even while enzymes are enormous in comparison to their substrates, only a tiny fraction of them are actually engaged in catalysis. The catalytic site is the location where catalysis takes place. The binding site is another place in an enzyme structure where the substrate interacts or binds.
The active site of an enzyme is made up of the catalytic and binding sites. The enzyme’s allosteric site refers to the location where another molecule can attach and cause the enzyme to alter its shape, resulting in an increase or decrease in activity. Inhibitors and activators are the two main categories of chemicals that influence enzyme activity. Molecules that limit or reduce enzyme activity are known as inhibitors.
Activators are substances that stimulate or boost the activity of enzymes. Denaturants are another factor that can influence enzyme function (chaotropic agents). Denaturation of the enzyme occurs when it is exposed to temperatures and pH levels that are outside of its optimum range. The enzyme’s structure and catalytic capabilities are lost after denaturation. The structure of an enzyme regulates its function. An enzyme’s specificity is determined by its 3D structure.
Types of Enzyme
The processes that enzymes catalyse are generally categorised and named after them. The EC numbers were created by the International Union of Biochemistry and Molecular Biology as a nomenclature for enzymes. The specifics are as follows:
1. Oxidoreductases: catalyse the processes of oxidation and reduction.
2. Transferases: a functional group is transferred (e.g. a methyl or phosphate group)
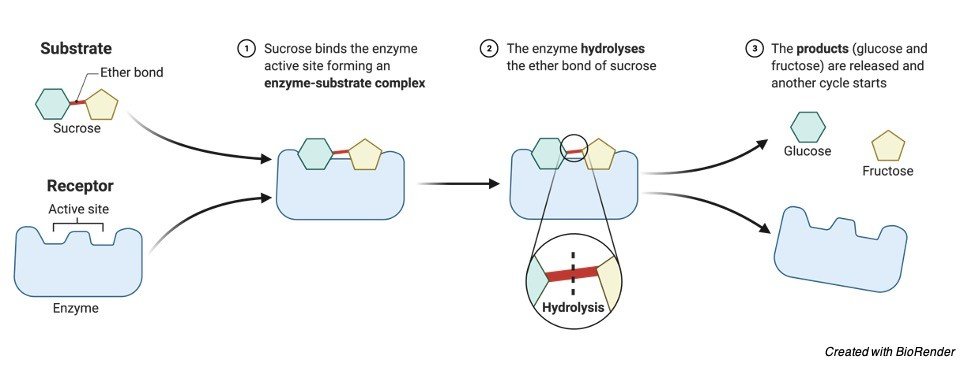
3. Hydrolases: these enzymes catalyse the hydrolysis of a variety of bonds.
4. Lyases: cleave bonds in a variety of ways other than hydrolysis and oxidation.
5. Isomerases: catalyse changes in isomerization within a single molecule.
6. Ligases: covalent bonds are used to connect two molecules.
Examples of Enzymatic Reaction
Transcription and translation are involved in the creation of enzymes through protein synthesis. Transcription and translation mechanisms within the cells produce an enzyme. Transcription is the process of creating an mRNA template from DNA, which encodes the sequence of amino acids in the form of a trinucleotide code and serves as a template for translation. Translation is the process by which amino acids are joined together in a precise order based on the genetic code’s instructions.
It is divided into four phases:
(1) activation (the amino acid is covalently bonded to the tRNA),
(2) initiation (the small subunit of the ribosome binds to the 5′ end of mRNA with the help of initiation factors),
(3) elongation (the next aminoacyl-tRNA in line binds to the ribosome with the help of GTP and an elongation factor), and
(4) termination (the ATG is released from the rib
On the newly produced proteinaceous structure, additional processes like as post-translational modification and folding will be conducted.
An enzyme, like any other catalyst, would be able to speed up a chemical reaction without disrupting its equilibrium. It denotes the absence of a catalyst in a reaction. An enzyme, on the other hand, is far more selective than non-biological catalysts. An enzyme must first attach to its substrate before it can catalyse a process. According to Daniel Koshland’s induced fit model, the enzyme reshapes as it interacts with its substrate, and the substrate may also change form somewhat, such that they finally fit into one another. By decreasing the activation energy, the enzyme accelerates a biological process.
It does this by either
(1) stabilising the transition state,
(2) offering an alternate route, or
(3) destabilising the substrate ground state.
For their catalytic activity, certain enzymes require non-protein substances known as cofactors. Organic or inorganic cofactors are also possible. Cofactors generally attach to the enzyme’s active site. The enzyme is called an apoenzyme when the cofactor is free, and a holoenzyme when the cofactor is bonded (however, holoenzyme also refers to an enzyme containing multiple protein subunits).
A coenzyme is a molecule that transfers chemical groups from one enzyme to another, such as hydride ions, phosphate groups, acetyl groups, and methyl groups. Coenzymes encompass NADH, NADPH, ATP, FMN (flavin mononucleotide), FAD (flavin adenine dinucleotide), TPP (thiamine pyrophosphate), and THF (tetrahydrofolate).
Biological Function of Enzyme
Enzymes are enzymes that act as biological catalysts. Chemical reactions are sped up by them. Enzymes are used in almost all metabolic processes to speed the conversion of substrates to products. When compared to a process without an enzyme, enzymes speed up the reaction rate by a million times.
Enzyme Citations
Share