What are Enzymes?
Proteins that facilitate chemical reactions are called enzymes.
Enzymes are biological catalysts that convert a substrate to a product.
They are typically globular proteins.
Isozymes catalyze the same reaction but with different kinetic parameters and possess different subunit composition.
Properties of Enzymes
They are active at very low concentrations within a cell.
They increase the rate of reaction (by decreasing the Activation Energy [Ea] of a reaction) towards equilibrium (they catalyze both the forward and reverse rxn) but they themselves aren’t altered in the process.
They do not change the nature of the products.
"Enzymes are typically globular proteins"
Although most enzymes are composed of proteins, many enzymes possess nonprotein components called as cofactors.
Cofactors can be metal ions or organic cofactors, coenzymes (ex. Acetyl CoA), which are usually derived from vitamins.
Vitamins are essential, can’t be produced by the body, organic molecules.
Coenzymes are divided into two (2) types:
a. Prosthetic group is an organic cofactor that is covalently bonded into an enzyme.
b. Cosubstrate reversibly binds to a specific enzyme, and transfers some chemical group to another substrate then reverted back to its original form by another enzymatic reaction.
ATP is an example of a cosubstrate type of coenzyme.
"Enzymes that require a cofactor but do not have one bound are called apoenzymes or apoproteins (are completely nonfunctional)"
Recall that lipoproteins contain a lipid core surrounded by phospholipids and apoproteins.
An apoenzyme together with its cofactor(s) is called a holoenzyme (this is the active form).
The equilibrium is not changed by enzymes, just the rate at which the equilibrium is reached.
In the process of catalyzing a reaction, the enzyme is not used up or permanently altered.
Enzymes don’t affect the direction of the reaction because they catalyzed both the forward and the reverse reaction at the same time by reducing the Ea.
Although enzymatic reactions use the same substrate and yields the same product as the uncatalyzed reaction, it produces a different intermediate at the transition state.
Like other chemical reactions, enzyme reactions are reversible, proceeding through the same set of reaction intermediates.
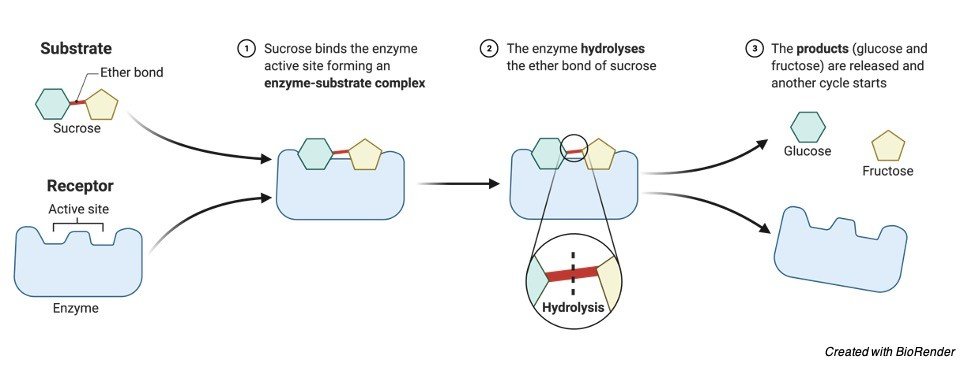
"The position on the enzyme to where the substrate binds, usually with numerous noncovalent bonds, is called the active site"
First the enzyme (E) and the substrate (S) bind to form the ES complex.
After conversion to transition states (ES* and EP*), the final product (P) is formed and then is released by the enzyme.
S + E ←→ ES ←→ ES* ←→ EP* ←→ EP ←→ E + P
An enzymatic reaction begins with the substrate binding at a specific location called the active site.
Think of an active site as a pocket into which the substrate fits.
The enzyme can bind the substrate only if it possesses the proper configuration.
Thus enzymes generally have a very high degree of specificity.
Many enzymes act only on one particular substrate.
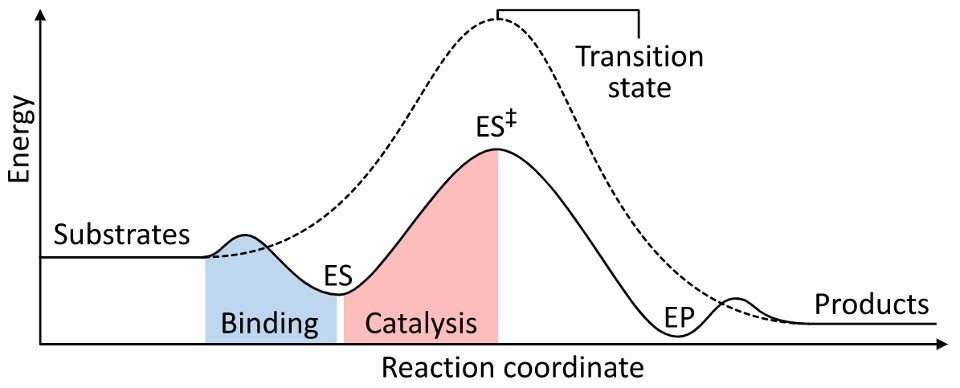
The equilibrium between Substrate (S) and Product (P) reflects the difference in the free energy of the ground states, ΔG.
On the other hand, the rate of the reaction depends on the height of the energy barrier between S and P.
The peak of the barrier represents the transition state of the reaction.
The difference between the energies of the ground states and the transition state is called the activation energy, ΔG‡.
To accelerate a reaction, an enzyme must lower the barrier for reaction – decrease ΔG‡
Once it binds the substrate, the enzyme induces a change in the molecular structure of the substrate, and when this occurs it makes the substrate more likely to spontaneously undergo more significant changes.
Enzyme Kinetics
They exhibit saturation kinetics; as the relative concentration of substrate increases, the rate of reaction also increases, but to a lesser and lesser degree until a maximum rate (Vmax) has been achieved.
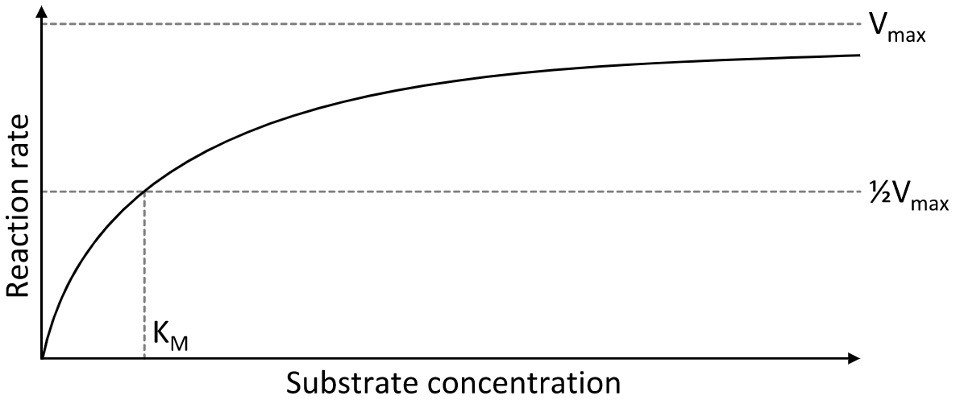
As we can see from this graph, once we have achieved saturation of the free enzymes the rate of reaction levels off and it cant go any faster.
"Vmax is proportional to enzyme concentration"
Turnover number: The number of substrate molecules one enzyme active site can convert to product in a given unit of time when an enzyme solution is saturated with substrate.
Km is the substrate concentration at which the reaction rate is equal to 1/2Vmax.
Km does not vary when the enzyme concentration is changed.
Is a good indicator of an enzyme’s affinity for its substrate.
Lower the Km the higher the affinity the enzyme has for a substrate.
Factors Affecting Enzyme Kinetics
1) pH:
Example: Pepsin prefers pH of below 2 while trypsin, active in the small intestine prefers pH between 6 and 7
2) Temperature: At first, as the temperature increases, the reaction rate goes up, but at some point, the enzyme denatures, and the rate of reaction drops off.
3) Salt Concentration
4) Hydrostatic Pressure
5) Substrate Concentration
First, environmental conditions can produce changes in weak bonds that can alter the 3-D structure of the enzyme.
For example: Warm temperatures can break bonds that are necessary to form the active site.
Second, environmental conditions can alter the ionization state of critical amino acids within the active site.
Third, environmental conditions can alter the ability of the enzyme to undergo structural changes necessary for catalysis.
Enzymes Citations
Share