Hypertonic Solution Definition
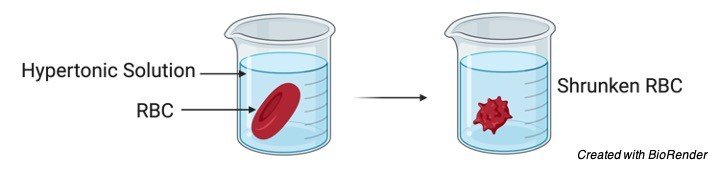
A solution which contains higher concentration of solutes comparing with another solution is referred to as hypertonic solution.
If a cell is placed in a hypertonic tonic solution it will shrink by allowing the water to move out.
When a hypertonic solution placed in an environment of low salt concentration or in a hypotonic solution then both solutions flow up and mix up with each other.
These two solutions will together form a single solution due to the passive exchange of ion and other molecules and gets equalized or isotonic in nature.
Effects of Hypertonic Solution
Since hypertonic solutions contains higher concentrations for e.g. cell, the water molecules move from inside of the cell to the outer environment by osmosis.
This allows the cells to decrease in its internal pressure and results in shrinking of the cell.
Examples of Hypertonic Solutions
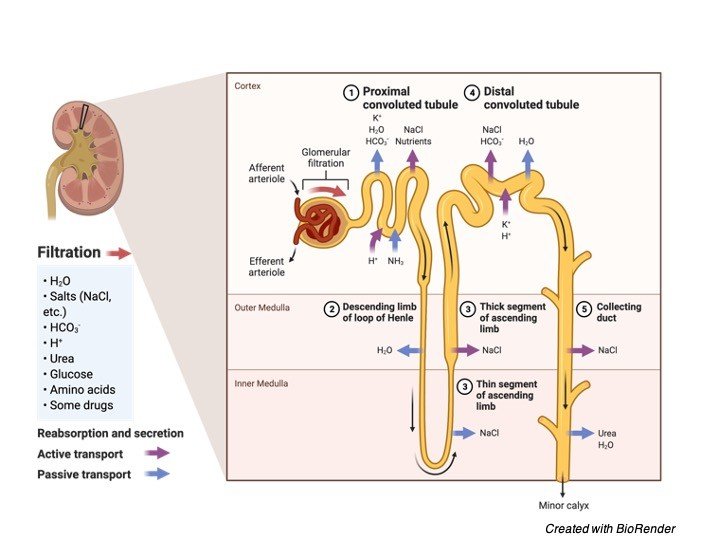
A. Human Kidney
Hypertonicity is generally maintained in human kidneys to regulate the amount of water in the body.
These are regulated by special proteins situated in the brain known as osmoreceptors, which also helps in maintaining the molecularity in the external environment of the cell.
When there is no water in the blood to dilute the solutes, Hypothalamus of brain releases hormones which play a key role in maintaining the permeability of the surface membrane of the kidney.
This helps the kidneys to reabsorb the water and add back to bloodstream which it has already excreted, and blood comes back to the normal isotonic state and regular process continues.
B. Sea Turtles
We all know that salt water is more hypertonic as compared with fresh water.
Sea turtle mostly live-in hypertonic regions where hypertonic solutions will dehydrate the turtles and the allows the body to balance different osmolarity.
Hence to overcome these obstacles sea water mammals and other aquatic species including sea turtles develops a unique pathway to remove excess salts through salt glands, which provides an internal environment with less or no loss of excess amount of water to the environment.
C. Plants
Generally, plants are adapted themselves to live in a hypotonic environment but some plants are adapted to live in a hypertonic solution.
Plants which live near marshy and in mangrove areas contain a high concentration of salts which allows the soil to become saturated with these salts.
But most of the plants do not survive to this habitat, but a special type of plant species known as Halophytes has evolved to overcome this obstacle by lowering the water potential of the root cell and to allow water to enter the cells.
This helps the cells to store the excess salts in roots and transports the salts to leaves where they can be excreted through their respective glands.
D. Animal Cells
As we all know plasma membrane separates a content of a cell from the external environment, which also helps in transporting the solutes across.
It also contains special protein channels known as aquaporins which allow the water to flow freely across the membranes.
In such cases energy is needed for the cells for active movement of solutes into and out of the cell.
Many solutes and cells became a hypertonic solution comparing it with the environment. In such conditions the cells without the cell wall will burst.
And in such cases the solutes in the outer environment will become hypertonic and here the water moves out of the cell against the concentration gradient of solutes which initiates the movement of solute from lower to higher concentration.
Generally, the organisms which regulate the osmolarity of their cells are known as osmoregulators.
In such cases the cells try to maintain their cytoplasm as a hypotonic solution comparing it to the environment.
During these cases structural changes occur which allows the water to flow freely through cells and allows it to participate in many of the necessary reactions.
E. Plant Cell
When cell shrinks due to hypertonicity water loses from both vacuoles and cytoplasm in such cases the plasma membrane is being pulled away from the cell wall and the cells become less turgid.
F. Human Osmoregulation
A healthy human body contains same amount of water present in and out of the cells. But when we sweat a lot, we lose more water than sodium, which makes the extracellular fluid hypertonic and makes us dehydrated.
In such cases osmosis occurs between fluid and red blood cells which results in depletion of red blood cells and prevents them from transport of oxygen in and out of the cells.
These hypertonic dehydrations may occur from mild to severe where the mild hypertonic dehydration causes thirst, dry mouth and tiredness and severe hypertonic dehydration results in low pressure, poor functioning of kidneys and muscle cramps.
Hypertonic Solutions as a Treatment
Hypertonic solutions are often used in hospitals in administration of drips and injections which helps the patient to build up their fluid availability in their cells and it also cures oedema which draws water away from bloated tissues and back into the blood stream.
They also help in replacing electrolytes in the body of sick individuals who cannot consume food or liquid themselves through few hypertonic solutions.
Hypertonic solutions help babies who are at the risk of hypoglycemia ((low blood sugar).
Apart from medical usages Hypertonic solutions are also used in food industries to preserve food such as pickling of food in hypertonic conditions which limits the microbes to reproduce.
Hypertonic solutions also help in dehydrating the food and other substances which allows the water to pass through a membrane and helps in maintaining equilibrium.
Hypertonic Citations
- Induction of labor by intra-amniotic instillation of hypertonic solution for therapeutic abortion or intrauterine death. Obstet Gynecol . 1969 May;33(5):729-36.
- Hypertonic solutions in the treatment of hypovolemic shock: a prospective, randomized study in patients admitted to the emergency room. Surgery . 1992 Apr;111(4):380-5.
- The effect of hypertonic solution on the wet weight and contractions of rat uterus and vas deferens. J Gen Physiol . 1969 May;53(5):590-607.
Share