Lipids Definition
A lipid is any biological molecule that has low solubility in water and high solubility in nonpolar organic solvents.
Types of Lipids and Lipids Function
There are six major groups of lipids:
1. Fatty Acids
a. Building blocks for most, but not all, complex lipids
b. Usually an even number of carbons, maximum # of carbons in humans is 24
c. Oxidation of fatty acids liberates large amounts of chemical energy for the cell, and most lipids reach the cell in the form of fatty acids and NOT as triacylglycerols.
d. Can be saturated or unsaturated
Saturated Fatty Acids contain only single carbon-carbon bonds
Unsaturated fatty acids contain one or more carbon-carbon double bonds

Saturated Fatty Acid

Unsaturated Fatty Acid
2. Triacylglycerols
a. Commonly called triglycerides or simply fats and oils, are constructed from a three carbon backbone called glycerol, which is attached to 3 fatty acids
b. Their function is to store energy and may also provide thermal insulation and padding to an organism
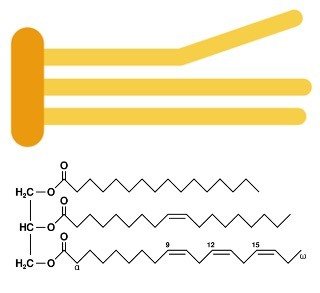
Example and structure of an unsaturated fat triglyceride

Fat in adipose tissue
c. Adipocytes, also called fat cells, are specialized cells whose cytoplasm contains almost nothing but triglycerides.
d. Lypolysis of triacylglycerols take place inside the adipose cells when blood levels of epinephrine, norepinephrine, glucagon or ACTH are high!!
3. Phospholipids
a. Are built from a glycerol backbone as well, but a polar phosphate group replaces one of the fatty acids.
b. The phosphate group lies on the opposite side of the glycerol from the fatty acids making this lipid polar on one end and nonpolar on the other end.

Phospholipids

Phospholipid arrangement in cell membranes.
c. This condition is called amphipathic, and makes phospholipids especially well suited as the major component of membranes
4. Glycolipids
a. Are similar to phospholipids, except that glycolipids have one or more carbohydrates attached to the 3-carbon glycerol backbone instead of the phosphate group.
b. Are also amphipathic
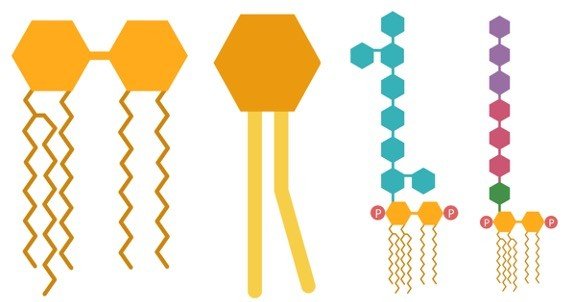
Structural arrangment of glycolipids
c. They are found in abundance in the membranes of myelinated cells composing the nervous system.
d. Also serve as markers for cellular recognition.
5. Steroids
a. are four ringed structures, which regulate metabolic activities.
b. Include some hormones, vitamin D, and cholesterol, an important membrane component.
6. Terpenes
a. include vitamin A, a vitamin important for vision
b. Their building block is the hydrocarbon isoprene, CH2=C(CH3)-CH=CH2 . Terpene hydrocarbons therefore have molecular formulas (C5H8)n
7. 20 Carbon Eicosanoids
a. Eicoanoids include prostaglandins, thromboxanes, and leukotrienes
b. Eicosanoids are released from cell membranes as local hormones that regulate, among other things, blood pressure, body temperature, and smooth muscle contraction.
c. Aspirin is a commonly used inhibitor in prostaglandin synthesis
d. Since lipids are insoluble in aqueous solution, they are transported in the blood via lipoproteins.
e. A lipoprotein is a biochemical assembly that contains both proteins and lipids.
f. It contains a lipid core surrounded by phospholipids and apoproteins.
g. Thus lipoproteins are able to dissolve lipids in its hydrophobic core, and then move freely through the aqueous solution due to its hydrophilic shell
h. Lipoproteins classified according to density.
The greater the ratio of lipid to protein, the lower the density.
i. The major classes of lipoproteins are chylomicrons, very low density lipoproteins (VLDL), low density lipoproteins (LDL – “bad cholesterol”), and high density lipoproteins (HDL – “good cholesterol”) – is arranged according to density.
Lipids Citations:
Share








