What is Hybridization?
The concept of hybridization was introduced by scientist pauling. He defined hybridization as redistribution of the energy of orbitals of individual atoms to give new orbitals of equivalent energy. This new orbitals formed are called hybrid orbital.
Features of Hybridization
• Number of hybrid orbitals = number of atomic orbital that get hybridized.
• Hybridized orbitals have equal energy and shape.
• Hybridization takes place with the orbitals of valence shell.
• Hybridisation does not take place in the isolated atom.
Types of Hybridization
sp Hybridization
When one s and one p orbital in same main shell mix together to give new hybrid, orbital called sp hybridised orbital. Forms linear molecules with 180 angle.
Example: all compounds of beryllium such as BeF2, BeCl2
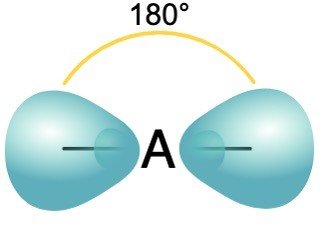
sp2 Hybridization
One s + two p orbitals of the same shell = 3 equivalent orbital , new orbitals formed are called sp2 hybrid orbitals.
Angle: 120
Shape: trigonal planar shape
Example: All the compounds of Boron such as BCl3
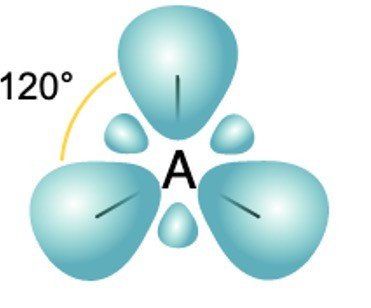
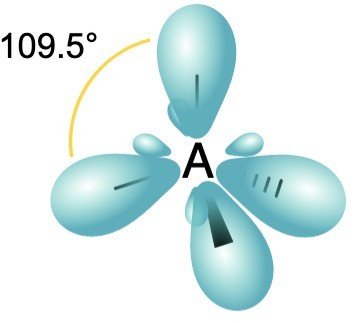
sp3 Hybridization
1 s orbital + 3 p orbitals of same shell = 4 new equivalent orbital. new orbitals formed are called sp3 hybrid orbitals.
Angle: 109°28’ with one another Shape Tetrahedral
Example: ethane (C2H6), methane.
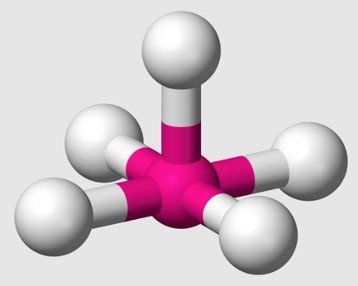
sp3d Hybridization
3p orbitals + 1d orbital = 5 orbitals of equal energy. Angle.
Equatorial orbitals: 3 hybrid orbitals lie in the horizontal plane inclined at an angle of 120 Axial orbitals.
The remaining 2 orbitals lie in the vertical plane at 90 degrees plane Shape : trigonal bipyramidal geometry
Example: Phosphorus pentachloride (PCl5)
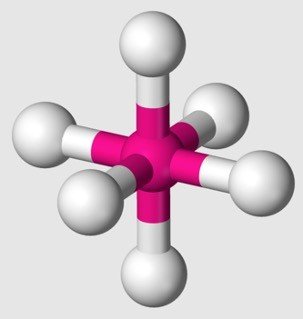
sp3d2 Hybridization
1s + 3p + 2d orbitals = 6 identical hybrid orbitals.
Shape: octahedron.
Angle: 90 degrees to one another.
Example: sulfur hexafluoride SF6
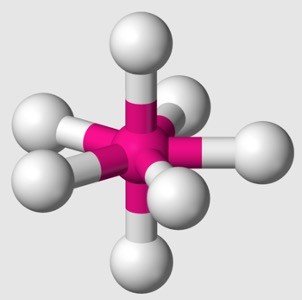
sp3d3 Hybridization
1s +3p+3d =5 orbital of same element mix and recast to form hybrid orbitals
Shape: pentagonal bipyramidal geometry
Example: IF7
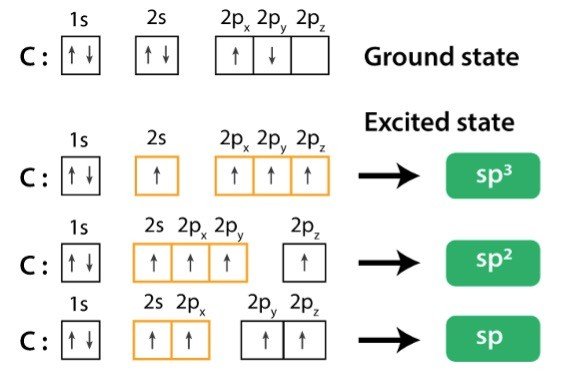
Concept of Hybridization of Carbon Atom
sp Hybridization: When Carbon is bound to two other atoms with the help of two double bonds or one single and one triple bond.
Example: Hybridization of CO2.
sp2 Hybridization: When carbon atom bonding takes place between 1 s-orbital with two p orbitals then the formation of two single bonds and one double bond between three atoms takes place.
Example: Hybridization of graphite
sp3 Hybridization: When the carbon atom is bonded to four other atoms.
Example: Hybridization of CH4 (Methane)
How to Determine Hybridization?
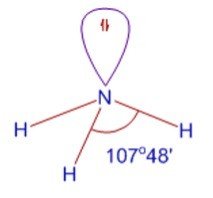
Hybridization of Nitrogen in Ammonia, NH3
Step 1: Write the Lewis structure
The valency of nitrogen is 3.
Thus, it forms 3 bonds with three hydrogen atoms.
There is also a lone pair on nitrogen.

Step 2: Calculate number of sigma (σ) bonds
Nitrogen in ammonia is bonded to 3 hydrogen atoms.
Number of sigma bonds are = 3.
Step 3: Calculate number of lone pairs
Number of lone pairs on nitrogen atom = (v – b – c) / 2
= (5 – 3 – 0) / 2 = 1 lone pair
There are 5 balance electrons in nitrogen atom before bond formation.
Step 4: Calculate steric number of nitrogen atom
Steric number = number of σ bonds + number of lone pairs
Thus, 3 + 1 = 4
Step 5: Give hybridization and shape of molecule
Nitrogen in ammonia is sp3 hybridization.
The shape is pyramidal.
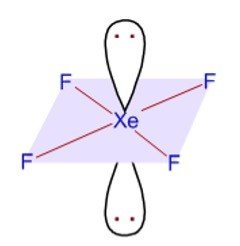
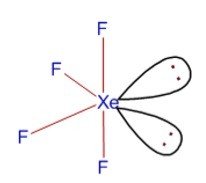
Hybridization and Shape of XeF4
Step 1: Calculate the number of sigma (σ) bonds
Number of sigma bonds formed by xenon = four as it is bonded to only 4 fluorine atoms.
The valency of fluorine = one.
Step 2: Calculate number of lone pairs
The number of lone pairs on xenon atom = (v – b – c) / 2 = (8 – 4 – 0) / 2
Step 3: Calculate the steric number of central atom
Steric number = number of σ-bonds + number of lone pairs = 4 + 2 = 6
Step 4: Allocate hybridization and shape of molecule
The hybridization is sp3d2.
Thus, Structure is based on octahedral geometry
Hence, Shape is square planar.
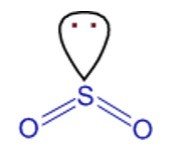
Hybridization and Shape of SO2
Step 1: Write the Lewis structure
Sulfur’s valency may be 2 or 4 or 6.
Oxygen’s valency = one.
Each oxygen makes two bonds with sulfur atom.
One is sigma bond and the second one is pi bond.
The total number of bonds formed by sulfur with two oxygen atoms = four.

Step 2: Calculate number of sigma (σ) bonds
The number of sigma bonds formed by sulfur atom = two
As it is bonded to two oxygen atoms.
Step3: Calculate number of lone pairs
The number of lone pairs on sulfur atom is = (v – b – c) / 2 = (6 – 4 – 0) / 2; Thus 1.
Number of valence electrons in sulfur is 6.
Total number of bonds including sigma and pi bonds is = 4.
Step 4: Calculate steric number of central atom
Steric number = no. of σ-bonds + no. of lone pairs = 2 + 1 Thus 3
Step 5: Give the hybridization and shape of molecule
The hybridization is sp2.
Structure is built on trigonal planar geometry with one lone pair.
Shape is = angular.
Steric Number
If steric number is 4, then it is sp3
If steric number is 3 then it is sp2
If steric number is 2 then it is sp
C1 – SN = 3 (three atoms connected), thus sp2
C2 – SN = 3 (three atoms connected), thus sp2
O4 – SN = 3 (1 atom + 2 lone pairs), thus sp2
O5 – SN = 4 (2 atoms + 2 lone pairs), thus sp3
C6 – SN = 4 (4 atoms), thus it is sp3
C7 – SN = 4 (4 atoms), thus it is sp3
N8 – SN = 4 (3 atoms + 1 lone pair), thus it is sp3
C9 – SN = 2 (2 atoms), thus it is sp
C10 – SN = 2 (2 atoms) thus it is sp
Hybridization Citations
Share