What is Uracil
Uracil is one of the nucleobases in the RNA, such as adenine, Guanine and cytosine where as in all living beings that contains DNA, lack uracil in its nucleotide base pairs.
It has adenine, thymine, Guanine and Cytosine, where adenine pairs up with thymine and guanine pairs up with cytosine, in case of RNA, thymine is replaced by the base pair uracil.
Uracil is also said to be the demethylated form of thymine. Uracil combines with the adenine through two hydrogen bonds.
Generally, Uracil is considered as one of the natural derivatives obtained from pyrimidine.
The term uracil was first coined by a German Chemist named Robert Behrend during his attempt of synthesizing derivatives in the uric acid. But it was first discovered by Albert Ascoli in the year 1900 during the process of isolating yeast nuclei by hydrolyzing.
Uracil have also been found in bovine thymus, spleen, herring sperm and in germ of wheat. It is basically a planar, unsaturated compound which has the ability to absorb light.
Based on the carbon ratios present in the organic compounds,12C/13C is considered as isotopic ratio; there are observed to be in the Murchson metrorite.
It also said that Uracil and Xanthine are mostly related molecules. and they have the ability to form during extraterrestrials.
On other analysis during the collection of data from orbiting the Saturn. It is also observed that Titan’s surface may also contain an uracil.
Properties of Uracil
Usually in the nucleic acid RNA, Uracil combines with adenine and it is further replaced by thymine during the transcription of DNA.
This evolutionary substitution of thymine instead of uracil increases the stability of the DNA. And it improves the efficiency of DNA replication.
Uracil pairs with adenine with the help of two hydrogen bonds. When this base pair occurs uracil act as a both donor and acceptor of a hydrogen bond.
Where as in RNA Uracil binds with the ribose sugar to form a ribonucleoside uridine. When a phosphate attaches to uridine, it results in the production of 5’-monophosphate.
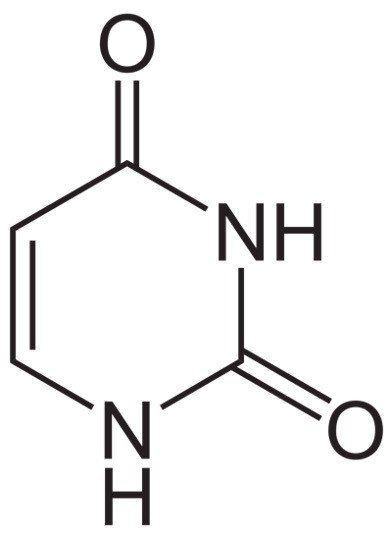
Uracil undergoes the tautomeric shifts of amide-imidic acid because lack of nuclear stability of the molecule leads to the unformal aromaticity during cyclic-amidic stability.
Here the tautomer of amide is referred to as lactam in structure and the imidic tautomer is referred to as lactim in structure.
These tautomeric forms are mostly predominant only at the ph. of 7. This lactam structure is the basic and common form of uracil. Uracil recycles itself to form nucleotides by undergoing series of phospho- ribosyl transferase reactions.
Degradation of these reactions results in the formation of substances like aspirate, carbon dioxide and ammonia. Uracil is generally considered as a weak acid.
Uracil and DNA
Uracil is most rarely found in the DNA. Because it often undergoes evolutionary changes which helps in increasing the stability through generations.
Because in cytosine diamines spontaneously produces uracil. By the process of hydrolytic deamination.
So, it can be said that if any organism uses uracil in DNA, the cytosine deaminates and leads to the formation of thymidine.
During the process of synthesis of DNA, the deamination of cytosil occurs which leads to the formation of uracil. During this process uracil-DNA bases excises from the double stranded DNA.
This enzyme recognises and cut the both types of uracil, where as the one is formed due to the cytosine deamination and the other would trigger unnecessary and unappropriated repair processes.
During the process of evolution, this is been solved by methylating uracil. This methylated uracil is being identical to thymine; hence this hypothesis states the reason for thymine being replaced by uracil.
So, the cells continue to use Uracil in RNA and it is not used in DNA, because RNA lives shorter than that of DNA.
If any potential errors occur in case of uracil, it leads to the damage in a particular strand.
There is also no evolutionary pressure in the RNA, to replace uracil with complex thymine’s, Some DNAs which consists of uracil still exists in some cases such in; DNA of several phages, end pterygote development, Hypermutations occurs during the synthesis of vertebrate antibodies.
Uses of Uracil
Uracil helps our body to carry out the synthesis of many enzymes which are necessary for the cell to function while bonding with ribose’s and phosphates.
Uracil serves as allosteric regulator and coenzyme for the reactions in plants and animals. UMP (Uridine Monophosphate) helps in controlling the activity of carbamoyl phosphate synthesis and in aspirate transcarboxylase in the plants while UDP and UTP helps in regulating the Cause II activity in the animals, where as UDP-glucose regulates the conversion of glucose into galactose in the organs such as liver and other tissues in the process of carbohydrate metabolism.
Uracil is also involved in the in the biosynthesis of polysaccharides and in the transportation of sugars containing aldehydes.
Uracil is also important in the detoxification of many carcinogens which is found in the cases of people who consume tobacco and smoke.
Uracil is also required in the process of detoxifying the drugs such as cannabinoids and other morphines.
If the body faces extreme deficiency of folates, it increases the risk of cancer, because the deficiency of folate the increases the ratio of deoxy uridine monophosphate or deoxythymidine phosphate and leads to misincorporation into the DNA which in turn results in low production of DNA.
Uracil has also been used in drug delivery in pharmaceuticals and also in medicines.
Uracil can also be used to determine the microbial contamination of tomatoes by indicating the lactic acid contamination in the fruit.
The mixtures containing uracil are most commonly use to test the reverse phase of HPLC columns.
Uracil Citation
- Uracil within DNA: an actor of antiviral immunity. Retrovirology . 2008 Jun 5;5:45.
- Uracil DNA glycosylase uses DNA hopping and short-range sliding to trap extrahelical uracils. Proc Natl Acad Sci U S A . 2008 Aug 5;105(31):10791-6.
- Therapeutic potential of uracil and its derivatives in countering pathogenic and physiological disorders. Eur J Med Chem . 2020 Dec 1;207:112801.
Share