What is ATP Synthase?
The ATP synthases are multiprotein buildings found in the energy-transducing films of microorganisms, chloroplasts and mitochondria.
They utilize a transmembrane protonmotive power, p, as a wellspring of energy to drive a mechanical rotating instrument that prompts the compound union of ATP from ADP what’s more, Pi.
Features of ATP Synthase
Their general design, association and unthinking standards are generally grounded, however different highlights are less surely knew.
For instance, ATP synthases from microbes, mitochondria and chloroplasts contrast in the systems of guideline of their movement, and the sub-atomic bases of these various instruments and their physiological jobs are as it were simply starting to arise.
Another essential component coming up short on a atomic depiction is the way revolution driven by p is produced, also, how pivot sends energy into the synergist locales of the protein to create the venturing activity during turn.
One amazing and deficiently clarified derivation dependent on the balance of c-rings in the rotor of the protein is that the measure of energy needed by the ATP synthase to make an ATP atom doesn’t have a general worth.
ATP synthases from multicellular living beings require the least energy, though the energy needed to make an ATP atom in unicellular living beings and chloroplasts is higher, and scope of qualities has been determined.
At last, the proof is developing for different jobs of ATP synthases in the internal films of mitochondria.
Here the chemicals structure supermolecular buildings, perhaps with explicit lipids and these buildings likely add to, or indeed, even decide the development of the cristae.
Structure of ATP Synthase
They comprise of two major utilitarian spaces, a layer extraneous F1 area furthermore, a film inherent Fo area consolidated by focal furthermore, fringe stalks.
The F1 area is the reactant part of the compound where ATP is shaped from ADP and Pi.
The Fo area contains an engine, which produces revolution utilizing the potential energy put away in p.
Schematic Representation of ATP Synthase
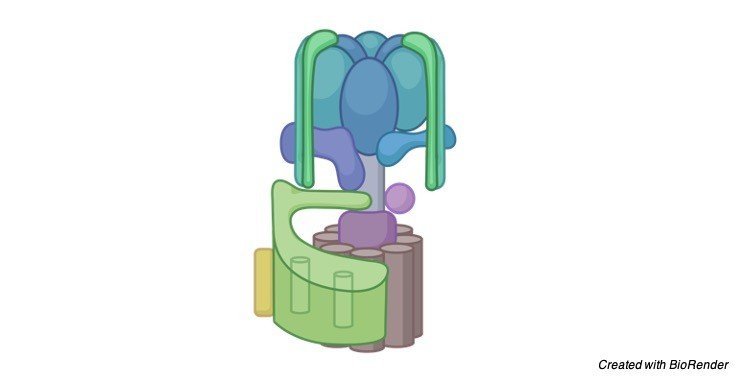
The rotational energy of the engine is communicated to the synergist area by the focal tail, which is joined straightforwardly to the turning engine.
The fringe tail interfaces the α3β3 area to subunit an in the Fo space, so together they structure the necessary stator of the compound.
The F1 area of the ATP synthase is a get together of five globular proteins, α, β, γ , δ and ε, with the stoichiometry α3β3γ 1δ1ε1.
The joined atomic mass of these subunits is around 350 kDa. In the α3β3γ 1 subassembly, which is normal to all ATP synthases, the three α-and the three β-subunits structure an around circular α3β3 structure around 100 A˚ (1 A˚ = 0.1 nm) in distance across, with the six subunits masterminded in shift around a prolonged α-helical design in the γ – subunit.
This piece of the γ – subunit is totally encompassed in the α3β3 area and involves its focal pivot.
In this way the α3β3 structure takes after an orange made of six fragments orchestrated around the focal substance tail of the organic product.
The rest of the γ – subunit projects roughly 30 A˚ past the α3β3 space and structures part of a ‘foot’ that joins the α3β3γ 1 complex solidly to the Fo area.
This connection is expanded by another protein, known for authentic reasons as the ε-subunit in microorganisms and chloroplasts, and as the δ-subunit in mitochondria.
Notwithstanding their various names, these ε-and δ-subunits are collapsed and presumably tie in a comparable way, and their capacities are presumably related likewise, in spite of the fact that they are not indistinguishable.
In mitochondria, the connection of the foot of the γ – subunit to the Fo space is increased by a subsequent subunit.
Referred to, confusingly, as the ε-subunit, this mitochondrial protein has no partner in bacterial and chloroplast catalysts.
The γ – subunit, what’s more, the related ε-subunits in the bacterial and chloroplast proteins, and the related δ-and ε-subunits in the mitochondrial protein, are bound immovably to one finish of a hydrophobic barrel shaped design made of a ring of csubunits in the Fo film space of the chemical.
This chamber, along with the γ – subunit and its related ε-and δ-subunits, shapes the rotor of the chemical.
Function of ATP Synthase Beyond Oxidative Phosphorylation
Throughout the long term, proof has gathered in microorganisms and mitochondria that respiratory buildings, including the ATP synthase complex, are coordinated into super-and supramolecular buildings.
Comparable perceptions have been made about photosynthetic buildings in the thylakoid layers of chloroplasts. In the internal layers of mitochondria, dimers of the ATP synthase are shaped by the relationship of their e-subunits in their film areas.
These dimers structure strips, or straight columns, which are confined at the locale of most noteworthy shape along the edges of the cristae.
It is well realized that cardiolipin (diphosphatidylglycerol) is fundamental for the action of a coupled ATP synthase complex.
Cardiolipin atoms have been proposed to tie in the area of a completely trimethylated lysine buildup at position 43 of the c-subunit.
This trimethylated lysine buildup is situated in the headgroup district of the bilayer , and its essence would hamper the limiting of the headgroups of phospholipids, giving destinations to cardiolipin to tie specially.
The ADP–ATP translocase, another plentiful part of the inward layers of mitochondria, additionally contains a trimethyl-lysine buildup, which is likewise in the headgroup area of the layer and would probably have a comparable inclination for restricting cardiolipin atoms.
The suggestion that these trimethyllysine buildups mark cardiolipin-restricting destinations is borne out by atomic elements recreations, and the trimethylated lysine buildup in subunit c is moderated in all vertebrate furthermore, invertebrate ATP synthase edifices that have been inspected, yet not in parasites or microorganisms.
The cardiolipin particles bound to the c8-rings of mitochondrial ATP synthase may balance out the c8- rings to assist them with enduring the rotational force to which they are oppressed.
They may likewise assist with greasing up the pivot of the ring in the film climate. Cardiolipin has been identified additionally in relationship with subunit a.
It is realized that around 75 % of cardiolipin in ox-like heart mitochondria is related with the network pamphlet of the internal layer, and the relationship of cardiolipin with subunit c and the ADP–ATP translocase would contribute to this nearby focus.
In the event that those cardiolipin particles related with ATP synthase buildings were roughly cone-molded, they would in general give the cristae a negative ebb and flow, the inverse to what in particular is noticed.
Consequently, the cardiolipin particles related with ATP synthase are bound to be leveled to the surfaces of the proteins in the layer area of the compound, and their job is probably going to be explicit to the activity of the ATP synthase itself.
ATP Synthase Citations
- Cardiolipin puts the seal on ATP synthase. Proc Natl Acad Sci U S A . 2016 Aug 2;113(31):8568-70.
- Opposite rotation directions in the synthesis and hydrolysis of ATP by the ATP synthase: hints from a subunit asymmetry. J Membr Biol . 2015 Apr;248(2):163-9.
- A novel chloroplast super-complex consisting of the ATP synthase and photosystem I reaction center. PLoS One . 2020 Aug 20;15(8):e0237569.
Share









