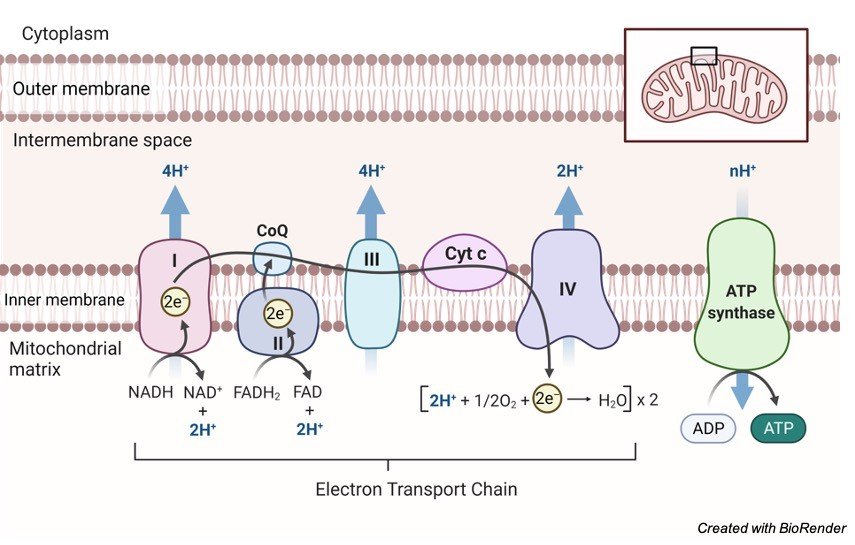
Mitochondrial Electron Transport Chain
Electron Transport chain is a series of catalysts with rising redox potential.
The basic mechanism involves collection of the reduced equivalents either hydrogen atom or electrons from the substrates and transferring them in a sequential order to get oxidised with the oxygen molecule resulting in formation of water and energy in the form of ATP.
The whole process is also called as redox reaction or respiratory chain.
The electron carriers are assembled in the form of chain within the four membrane-bound enzyme complexes, which are embedded in the inner mitochondrial membrane.
The main advantage of this system is that the electrons transports in a stepwise manner from substrate to oxygen, thus, generating enthalpy slowly in a systematic way preventing sudden burst of energy making the process efficient and well controlled.
Component of Electron Transport Chain
The electron transport chain is composed of:
A. Hydrogen and electron carriers
B. Four membrane-bound enzyme complexes
A. Hydrogen and Electron Carriers of the Electron Transport Chain
1. Nicotinamide Adenine Dinucleotide (NAD): It is a co-enzyme that carries hydride ion (H-) and thus known as a hydride carrier.
It gains 2 hydrogen atoms from the substrate of Citric acid cycle like isocitrate, malate, B-hydroxy acyl CoA and B-hydroxy butyrate and form NADH molecule.
In the process, one electron releases which reduces NADH to NADH+ while passing its hydrogen to flavoprotein containing FMN and iron sulphur protein (FeS).
2. Flavoproteins (FAD and FMN): In the meanwhile, flavoproteins like FAD and FMN both serves as hydrogen acceptor wherein they tightly bound the hydrogen atom.
Thus, directly preventing its reduced form to react with oxygen.
In general, the types of flavoprotein receiving hydrogen passes them to coenzyme Q.
For example, flavoprotein Fp1 receives 2 hydrogen atoms from reduced NAD+, while flavoprotein Fp2 receiving the same from various substrates like succinate, acyl CoA and choline.
3. Ubiquinone: It is also known as Coenzyme Q that contains quinone ring. Coenzyme Q is the most common ubiquinone that shows similarity with vitamin K.
It is lipid soluble with small molecular size thus responsible for free mobility of the molecule in the inner membrane of mitochondria.
It is the basic carrier of the hydrogen atoms, wherein ubiquinol carries 2 hydrogen atoms while semiquinone carries only one.
These molecules connect flavoprotein with cytochrome b, where flavoprotein carries 2 hydrogen atoms while cytochrome b carry one.
Reduction of coenzyme Q leads to passage of electrons to cytochrome b while releasing 2H+ into the mitochondrial matrix.
The enzymes responsible for the oxidation of ubiquinol involves:
a): Conduction of electrons to cytochrome c is mainly responsible by ubiquinol (coenzyme Q) dehydrogenase along with coenzymes cyt b, FeS protein and cyt c1.
b): Another further transfer from cyt c to oxygen is carried out under the presence of cytochrome oxidase along with cyt a and cyt a3 as coenzymes.
4. Cytochromes are mainly responsible to carry electron from coenzyme Q to oxygen. Thus, exhibits the important role of electron carrier.
They are categorized with the letter a, b and c.
The core molecule for all the cytochromes are haemoproteins however they diverge in their redox potential.
The iron of cytochrome frequently changes their state from oxidation (Fe3+; ferric state) to reduction (Fe2+; Ferrous state) during their normal physiologic action, while that of haemoglobin remains in the reduction state.
Being mobile components, both coenzyme Q and cytochrome c transport reduced equivalent from the other fixed components.
5. Iron Sulfur Clusters(FeS): One additional component found is iron sulfur proteins also known as FeS or non-heme iron.
It is often related with flavoproteins and cytochrome b wherein iron is interchangeably exchange between flavoprotein and cytochrome b resulting in redox reaction.
B. Enzyme Complexes of the Electron Transport Chain
In the inner mitochondrial membrane four enzyme complexes are arranged which transport electrons. The following are the enzyme complexes observed:
Complex I known as NADH dehydrogenase (NADH-ubiquinone oxidoreductase) It is a flavoprotein wherein FMN and FeS protein are the coenzymes.
It facilitates transport of hydrogen atoms from NADH to ubiquinone in the form of NADH+ H+.
Complex II is known as Succinate dehydrogenase (succinate-ubiquinone oxidoreductase).
It is also a flavoprotein wherein FAD and FeS protein are the coenzymes.
It transfers hydrogen atoms from succinate to ubiquinone.
Complex III is known as Ubiquinol dehydrogenase (ubiquinol-cytochrome c oxidoreductase).
It facilitates transport of electrons from ubiquinol to cytochrome c with the help of coenzymes cyt b and cyt c1.
Complex IV is known as Cytochrome oxidase (cytochrome-oxygen oxidoreductase).
It facilitates transport of electrons from cytochrome c to oxygen with the help of coenzymes cyt a and cyt a3.
Moreover, there is a fifth complex (Complex V) known as ATP synthase and is facilitates the conversion of ADP to ATP using inorganic phosphate.
Electron Flow in Electron Transport Chain
The oxidation of substrates with the help of NAD+ or FAD results in production of hydrogen atoms that can pass into the electron chain transfer which further successfully transferred to oxygen to generate energy and water.
The hydrogen atoms cannot be accepted directly by the cytochrome b.
Thus, Coenzyme Q ionized the hydrogen atoms to release hydrogen ions and electrons.
The electrons will reduce the heme group from the cytochrome B, with this starts the transfer chain of electrons from cyt c1, cyt c, cyt a to cyta3.
Finally, the electrons are transported with the help of cytochrome oxidase towards oxygen to form ionic oxygen.
ATP Yield in Electron Transport Chain
The ATP count varies with the catabolism of glucose. Moreover, FAD+ molecules can transport fewer ions resulting in fewer ATP molecules.
In addition to this, the intermediate components of glucose can be used for different purposes resulting in variation in the ATP count.
Summary of Electron Transport Chain
The electron transport chain plays significant role in the aerobic respiration where the free oxygen accepts the last electron produced after glucose catabolism as a result from intermediate compounds.
The structural configuration of electron transport chain showed four large, multi-protein complexes penetrated in the inner membrane of mitochondria along with this 2 small diffusible electron carrier are present transferring electrons between them.
During the transfer of the electrons, small amount of energy is lost against the chemiosmosis.
Thus, high-energy electrons are donated by the NADH or FADH to compensate the energy to finish the chain.
Water and ATP are the end products of the electron transport chain.
Moreover, various intermediate products of the tricarboxylic acid cycle can be averted into the anabolism of other biochemical molecules which may serve as the benefactor for glucongenesis.
Electron Transport Chain Citations:
- Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol . 2020 Oct;37:101674.
- Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol . 2004 Jun;7(3):254-61.
- The mitochondrial respiratory chain. Essays Biochem. 2010;47:1-23.
- Bacterial electron transport chains. Annu Rev Biochem . 1988;57:101-32.
- Kinetic modeling of the photosynthetic electron transport chain. Bioelectrochemistry . 2001 Jan;53(1):35-53.
- Electron transport chain defects in Alzheimer’s disease. Neurology . 1995 Mar;45(3 Pt 1):599-600.
- Bioflavonoid effects on the mitochondrial respiratory electron transport chain and cytochrome c redox state. Redox Rep . 1999;4(1-2):35-41.
- Electron transport in xanthine oxidase. A model for other biological electron transport chains. Biophys J . 1976 Aug;16(8):939-51.
Share