-

Lock and Key Model: Definition, Function, and...
Continue ReadingLock and Key Model Definition
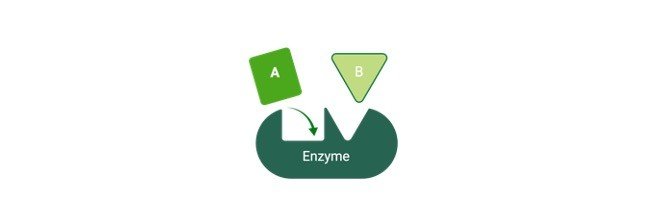
A paradigm for enzyme-substrate interaction suggests the enzyme and substrate have complimentary geometric forms that fit perfectly together. Enzymes have a high level of specificity. Before they may catalyse a chemical process, they must first attach to a particular substrate.
At the moment, two models are used to describe enzyme specificity:
(1) The lock and key model
(2) The induced fit model
The enzyme-substrate interaction in the lock-and-key paradigm implies that the enzyme and the substrate have complimentary geometric forms that fit perfectly together.
Only the right size and form of the substrate (the key) would fit into the active site (the key hole) of the enzyme, similar to a key into a lock (the lock).
According to the induced fit model proposed by Daniel Koshland in 1958, the active site changes until the substrate is entirely bonded to the enzyme’s active site, at which time the final shape and charge are established.
The induced fit model, in contrast to the lock-and-key paradigm, demonstrates that enzymes are very flexible structures.
Emil Fischer proposed the lock and key model hypothesis in 1894, which demonstrates the great specificity of enzymes. It does not, however, explain the enzymes’ ability to stabilise the transition state.
Lock and Key Model Citations
Share
Similar Post:
-

Skeletal System: Definition, Function, and Examples
Continue ReadingSkeletal System Definition
The major role of the skeleton is to provide structural support and protection, and it is made up mostly of bones, cartilage, ligaments, and tendons.
An organ system (or simply a system) is a collection of organs that work together to achieve a certain purpose. The integumentary system, lymphatic system, muscular system, nervous system, reproductive system, urinary system, respiratory system, skeletal system, and immunological system are the organ systems in humans and other animals.
The skeletal system is a set of organs that act as the structure for an organism’s body. Other structures such as bones, cartilage, ligaments, and tendons are produced in the connective tissues.
The skeleton refers to all of an organism’s bones and cartilage. It might be either an exoskeleton or an endoskeleton. The skeletal structures of an endoskeleton are located within the body. An exoskeleton is a form of skeleton that exists outside of an organism’s body.
An endoskeleton is found in most animals. The skeleton in humans is of the endoskeleton type, with 206 bones. The smallest bones are located in the middle ear, whereas the femur is the biggest bone. Crabs, shrimp, insects, and a variety of other invertebrates have exoskeletons.
Skeletal System Citations
Share
Similar Post:
-

Amphipathic Molecules: Definition, Types, and Examples
Continue ReadingAmphipathic Definition
Amphipathic is a Greek word, where amphis means “both” and pathy means “feeling”. Compounds which are soluble in water as well as non-soluble are termed as Amphipathic. Thus, they are hydrophobic as well as hydrophilic. They play a vital role as they are important for micelle formation and membrane formation. Thus, plasma membrane makes a barrier so that only selective material can pass through. Thus, maintaining homeostasis and concentration level.
What is Amphipathic Molecule?
Amphipathic molecules possess both hydrophobic and hydrophilic parts. In the hydrocarbon part is a large molecule of carbon which is non- water loving and lipophilic. There is a charge which can be of cations or anions or no charge present on the hydrophilic part with functional groups that are polar. As anions are negative charged the groups present would be sulfonates, phosphates, carboxylates and phosphates. Positive charged groups is ammonium. Thus, the amphipathic molecule has hydrophobic as well as hydrophilic parts.
Amphipathic Characteristics
As the amphipathic molecule possess both hydrophobic and hydrophilic parts, it will have opposite function such as with polar molecules hydrophilic portion will interact. Hydrophobic end will interact with non-polar molecule. Thus, to separate amphipathic molecule into two parts, there should be aqueous as well as non-polar organic solvent.
Examples of Amphipathic Biomolecules
Many amphipathic biomolecule are saponin, cholesterol, lipid, glycolipid, phospholipid and proteins. The amphipathic compound is called the amphiphile.
i. Amphipathic Proteins
Polar and non-polar amino acid sequences are present on these proteins. For example, polar amino acid will be present on hydrophilic end which is formed from protein and on the hydrophobic end non-polar amino acid. In the membranes presence of membrane proteins. As they are amphipathic their hydrophobic portion allows them to interact with the nonpolar region and simultaneously the polar region with the hydrophobic end.
A protein helix with opposing faces is called as amphipathic helix. In the long axis of helix, face in the same direction is called as hydrophilic while the face in the opposite direction is hydrophobic. Proteins hydrophobic and hydrophilic domains can be separated. Protein-protein interaction and self- interaction is possible. Ion channel membrane proteins, apolipoprotein and lung surfactant proteins are example of protein with confirmation.
ii. Phospholipids
b) This molecule are lipid molecule with phosphate group and two fatty acid. the lipid present is glycerol. To the phosphate, which is negatively charged is attached to a glycerol. The phosphate group has the hydrophilic head of phospholipid, which is bound to choline, serine, inositol thus forming phosphatidylserine, phosphatidylinositol and other phospholipid. The tail is made up of two fatty acid which is lipophilic hydrophobic phospholipid tail. There are phospholipid layers in plasma membrane.
As the head is hydrophilic it will interact with polar molecule, whereas the tail is hydrophobic it will interact with non-polar molecule. Thus, the presence of phospholipid in water will make the head exposed to the water and the tails away from the water. Thus, the plasma membrane structure of phospholipids is formed due to amphipathic nature of phospholipid. The heads face the plasma membrane exterior whereas the tails are present in the plasma membrane internally.
iii. Cholesterol
Cholesterol is made up of the hydrophobic hydrocarbon chain and hydrophilic hydroxyl group. It is present in the plasma membrane of animals. To the aqueous medium, hydrophilic head will interact and the to the hydrophobic tail, non-polar solvents will interact.
iv. Glycolipids
Glycolipids are found in the plasma membrane. They are constructed from hydrophilic sugar and attached to hydrophobic lipid tail. In the cell’s outer environment is the carbohydrate which interacts with other sugars and in the lipid, bilayer is the lipid present.
v. Bile- acids
They have a ring like steroid structure with side chain as hydroxyl group with four rings. Around the lipid droplet, bile acid salts will coagulate to form micelle. Thus, forming surfactant. Fats are emulsified so as to stop the coagulation of fats into larger fats.
vi. Saponins
They are present in plants. To keep the herbivore in distant plants release them. It is made up of a glycoside molecule which is hydrophilic and a hydrophobic side chain of steroid. In taste they are pungent and savoury.
Amphipathic Function
i. Membrane Formation
The amphipathic property makes it selectively permeable. An example is plasma membrane which is formed from biomolecules. In plasma membrane, phospholipids has taken a huge space, which have hydrophilic and hydrophobic parts, forming a lipid bilayer. In the lipid bilayer lies the phospholipid tail and the head is positioned at the exterior of the lipid bilayer.
The hydrophobic head and tails are positioned in such a way so as to facilitate movement. Polar molecules cannot pass through the membrane and they have to be modulated, thus, requires a transporter to transport polar molecules but non-polar small molecules can pass. With the hydrophobic lipid bilayer, membrane proteins can interact as they are also amphipathic. Thus, polar and charged molecules can pass through with the help of membrane protein and homeostasis is well maintained.
The movement of molecule is also controlled by the organelles present inside. Cholesterol is present in the animal cell’s plasma membrane and keeps the structure of animal intact and maintains the fluidity of the membrane and thus they do not contain cell wall. Other functions are movement of molecules, conduction of nerve, intracellular transport and signaling of cell. cell to cell interaction, stability are the functions of glycolipid, which is a plasma membrane component. Cell adhesion and cell recognition are the other functions.
ii. Micelle Formation
When the hydrophilic head are with the polar solvent and the hydrophobic head are positioned in the middle, it forms a cluster of molecules it is called micelle. Bile acid can form micelle, due to the amphipathic property. Shape is spherical. Lipid digestion is possible due to micelles in the bile acid. For better absorption, lipids are moved to the edge of the intestinal brush.
Amphipathic Citations
- Oral amphipathic peptides as therapeutic agents. Expert Opin Investig Drugs . 2006 Jan;15(1):13-21.
- Amphipathic, alpha-helical antimicrobial peptides. Biopolymers . 2000;55(1):4-30.
- Protein Amphipathic Helix Insertion: A Mechanism to Induce Membrane Fission. Front Cell Dev Biol . 2019 Dec 10;7:291.
- The Many Faces of Amphipathic Helices. Biomolecules . 2018 Jul 5;8(3):45.
- Amphipathic molecules modulate PIEZO1 activity. Biochem Soc Trans . 2019 Dec 20;47(6):1833-1842.
Share
Similar Post:
-

Absorption: Definition, Mechanism, and Examples
Continue ReadingAbsorption Definition
Absorption refers to the act or process of keeping light energy without reflection or transmission after passing through a material, as in the absorption of light by atoms, in other related fields such as physics. Absorption in chemistry refers to the process by which one material permeates another, such as a liquid penetrating or being absorbed by a solid.
Absorption Etymology
The word absorption comes from the Latin word absorptio, which means “to absorb.”
What is Absorption?
Absorption or integrating things into cells or across tissues and organs via diffusion or osmosis, as in nutritional absorption by the digestive system or medication absorption by the bloodstream. In a broad sense, absorption refers to the act or process of absorbing or assimilating something.
Absorption refers to the process of absorbing or assimilating substances into the cell or across tissues and organs in biology. Diffusion or osmosis are used to accomplish this. Absorption via the skin, for example, is one method for chemicals to enter the body. The act of absorbing some hazardous chemicals via the skin (also known as dermal absorption) is one of the ways they might enter the body (others include inhalation and ingestion).
For some medicines, dermal absorption can be utilised as a route of delivery. The absorption of digested food, particularly via the intestinal wall, is another type of biological absorption (especially in the small intestine). By diffusion or active transport, the digested food in the small intestine travels past the walls of the small intestine and into the blood vessels.
Absorption Citations
Share
Similar Post:
-
Biomes: Definition, Characteristics, and Examples
Continue ReadingBiome Definition
Biomes do not have a universal definition. However, through evaluating ideas, the biome definition in biology exposes the ecological reality. Biomes are the world’s great communities. They are categorised based on their unique qualities, such as climate, flora, soil, and animals. Biomes are important. They vary continually throughout history as a result of harm caused by human actions, for example. As a result, we should continue to preserve and conserve biomes.
Different ecological ideas, such as biomes and ecosystems, might be confusing to certain individuals. An ecosystem is a system of interactions between diverse living species in a given habitat, such as plants and animals. The primary distinction between a biome and an ecosystem is that the ecosystem is a subset of a biome. As a result, a biome will be made up of many ecosystems.
What is Biome?
A biome is essentially a vast habitat defined by numerous biotic and abiotic variables (e.g. temperature, precipitation, pH, light intensity, and so on). Biomes can be classified in a variety of ways. One of them is based on the weather, which might be hot, dry, chilly, wet, or humid. Biomes are developed as a result of physical climates, which impact the soil, precipitation, and fauna as a result.
Types of Biomes
The planet is divided into six biomes. Forest, desert, grassland, and tundra are the four terrestrial biomes, while marine and freshwater biomes are the two aquatic biomes. Temperature rainforest, tropical rainforest, taiga, and savanna are examples of different types of biomes.
Forests are essential habitats for a variety of biotic groups. They support a wide range of animals by providing habitat and food. As a result, a forest may contain a variety of microhabitats. Furthermore, forests contribute to the global capacity for climate buffering; hence, forest loss may result in significant changes in the local or global climate.
Because water is the most vital component of all living species, the marine and freshwater biomes are the most significant examples of biomes. It is a vital biomolecule in the human body. In addition, both marine and freshwater habitats support a large number of living species. Because the majority of the earth’s surface is covered by water, the oceans have a greater impact on the global climate than forests. The Earth’s hydrosphere (water component) has a vast number of photosynthetic planktons that create oxygen, which sustains the massive population of aerobic creatures that thrive in the oceans, seas, and freshwater, in addition to helping to regulate atmospheric temperature.
Despite being one of the most essential biomes on the planet, freshwater biomes are severely polluted. Overfishing, for example, destabilises these biomes and kills many species inside them.
Biomes are helpful for ecological research because they aid in the definition of ecosystem changes using remote sensing satellites. Biomes may also provide insight into the operation of ecosystems. Ecosystem production, plant function, and climate change are just a few examples.
Expert knowledge, as well as vegetation maps from various locations, and satellites are used to create the Earth’s biome map. Satellites like A-train are used to track the Earth’s weather, for example, by collecting satellite photos and doing remote sensing. The NASA Earth Observatory is a web-based archive of satellite images.
In terms of the numerous climatic thresholds that impact biome borders, satellite-based biome maps are more accurate and impartial. The world biome map aids in the comparison of various ecosystems in various parts of the globe. Various species and biological systems may exist in the same biome in different regions of the world. This diversity assists in the study of evolutionary and ecological processes in several areas.
The following biomes list shows the many sorts of biomes seen on the biomes map, along with additional biome facts that clarify biomes’ meaning:
I. Forests Biome
Forests are densely forested. They are home to a diverse range of animals, including birds, insects, and mammals. Tropical forests, temperate forests, and boreal forests are the three primary biomes of forests (Taiga). Due to their geographical location, these woods are subjected to a variety of climatic conditions. As a result, distinct forest types are categorised as follows:
i. Tropical Rainforests
Tropical rainforests resemble jungles in appearance. They live near the equator, so the weather is wet and hot all year. Tropical rainforests are home to a variety of species as well as a large number of trees that provide refuge for numerous animals while also contributing to oxygen generation and climate buffering capabilities.
ii. Temperate Forests
In temperate woods, all four seasons rotate throughout the year; leaves shed and fall in the fall, while trees are dormant in the winter. In addition, bears, deer, and woodpeckers can be seen in the winter.
iii. Boreal Forest (Taiga)
This biome is the world’s biggest terrestrial (land) biome. The presence of conifers (conical-shaped trees) characterises the Taiga biome. Winters in the boreal forests are cold, dry, and lengthy, and most birds migrate and animals hibernate. During the winter, certain creatures remain active and develop. As a result, they have hair or feathers to keep their bodies warm and can survive in frigid environments.
II. Deserts Biome
Deserts have the highest average temperature of any biome. During the winter, however, it gets quite frigid. The severe temperature swings lead to the existence of extreme habitats in the desert, where numerous species relocate to underground shelters to survive in more stable temperatures. Furthermore, animals and plants in desert biomes can usually survive for lengthy periods of time without water.
III. Tundra Biome
The tundra biome is cold and flat, with the lowest temperature of all biomes and poor soil nutrients, resulting in the presence of short plants such as moss, shrubs, lichens, and grasses that grow during the summer because a thick ice layer, known as permafrost, is present beneath the soil throughout the year. As a result, trees are unable to develop roots in the soil because the ice covering prevents them from doing so. During the summer, birds may be found breeding in the tundra, but they move south in the winter to warmer climates. During the winter, mammals such as mice dwell in tunnels beneath the snow.
The tundra biome is under grave danger as a result of climate change. The tundra ecosystem, its permafrost, and the animals that live there are all being disrupted by global warming.
IV. Grasslands Biome
Grasslands have no trees and are dominated by short to tall grasses. Because the weather is generally dry and warm, these regions do not receive enough rain to support tree growth. They do, however, get enough rain to produce certain plants, flowers, and grass. Grasslands are home to large animals that move in herds.
Temperate grasslands and savannah grasslands are the two types of grasslands (tropical grasslands). Near the equator, the savanna is common. Because they get seasonal rainfall, trees in the biome tend to grow alone or in groups. There are generally animals with lengthy legs that dwell there. They live in herds and may flee predators by running, for example.
Humans have had a significant impact on the grassland ecosystem. Most of the grasslands in the United States with fertile soil have been utilised for grazing livestock or crops. Various species were impacted, ranging from small creatures such as monarch butterflies to big animals such as elephants. Excessive hunting of big animals, for example, will disrupt an ecosystem’s biological equilibrium. For example, if grasses are lost, grazing animals such as zebras, which are a food supply for predators and carnivores in the region, may perish.
V. Freshwater Biome
Freshwater has a low salt content, around one percent. Rivers, streams, lakes, and ponds are examples of freshwater biomes.
VI. Marine Biome
The marine biome is the world’s biggest biome, covering about 70% of the globe. Among the world’s five major oceans are the Arctic Ocean, Southern Ocean, Indian Ocean, Atlantic Ocean, and Pacific Ocean. Because sea water has high salt concentrations, plants and animals in marine environments adapt by excreting surplus salt or boosting water absorption (homeostasis).
Biomes Examples
Forests encompass around one-third of the earth’s surface. They can be found in a variety of geological areas. Temperate woods, for example, can be found throughout Eurasia and eastern North America. Squirrels, deer, and bears are among the animals present in temperate woods. Tropical forests are another form of forest. They may be found in areas around the equator, such as Central America, Southeast Asia, and Sub-Saharan Africa. Large birds and harpy eagles are examples of creatures that thrive in tropical woods. Canada, Alaska, Scandinavia, and Siberia all have taiga (Boreal woods). The Boreal woods are home to deer, moose, and other big animals.
Deserts encompass roughly a fifth of the earth’s surface area and are classified into four types based on their temperature or location: hot, cold, coastal, and semiarid. Most of Africa’s continent is covered by the Sahara desert. It is well-known as an arid desert. The Moava desert, located in the southwest of the United States, is another desert habitat. Semiarid deserts span parts of North America, Asia, Greenland, and Europe; the Atacama desert of Chile is an example of a coastal desert in South America, and the Antarctic is a well-known cold desert.
There are two varieties of tundra, both of which may be found in high-latitude areas: alpine and arctic tundra. The alpine tundra is located at the tops of very high mountains, where temperatures drop dramatically at night. The arctic tundra can be found in Russia, Iceland, Canada, Greenland, Scandinavia, and Alaska, to the north of boreal forests on high landmasses.
Tundra animals generate fat layers to maintain their body temperature throughout the winter. To keep warm, they are coated in fur. Small mammals, such as ground squirrels, and big mammals, such as wolves, live on the tundra (e.g. caribou). Snowy owls, polar bears, arctic foxes, and wolves are tundra carnivores at the top of the tundra food chain. During the winter, they typically acquire white feathers or fur to help them blend in with the snow.
The majority of Africa, as well as parts of India, Asia, Australia, and South America, are covered with savannas. Away from the equator, temperate grasslands may be found in Argentina, Eastern Europe, North America, and Russia. There are no plants or trees in the temperate grasslands. Although savanna and temperate grasslands appear to be similar, they are distinct in numerous ways. Elephants, for example, are not found in the temperate grasslands of the United States, but they are prevalent in Africa’s savannas. Prairie dogs, on the other hand, are often found in temperate grasslands.
About 75% of the Earth’s surface is covered by freshwater and marine biomes. The major sources of freshwater running rivers and streams are rainwater or melting glaciers. Lakes and ponds are the immobile forms of freshwater that generally flow into an ocean or a lake. The seas are saltwater bodies that cover the majority of the earth’s surface. Marine biomes are home to a variety of living organisms. In the deep oceans, there is insufficient light to allow photosynthesis, so many animals rely on chemosynthesis to survive. Coral reefs are made up of calcium carbonate and develop in shallow seas. Because the waters are becoming more acidic and hotter, climate change has a significant impact on coral reefs.
You’re undoubtedly curious about the ecosystem in which we dwell. For example, if you live in California, your biomes are temperate forests in northern California, Redwood forests in northern California, and grassland in Western North America in the mountains. Furthermore, the desert biome may be found in a variety of locations.
3D biome models were created to offer a virtual look at each biome listed in the list of biomes using a computer, tablet, or phone in order to explore diverse biomes in different areas across the world.
Biomes Citations
- Ecological consequences of the expansion of N₂-fixing plants in cold biomes. Oecologia . 2014 Sep;176(1):11-24.
- The microbial ocean from genomes to biomes. Nature . 2009 May 14;459(7244):200-6.
- Paleobotany and Global Change: Important Lessons for Species to Biomes from Vegetation Responses to Past Global Change. Annu Rev Plant Biol . 2018 Apr 29;69:761-787.
- Biological nitrogen fixation across major biomes in Latin America: Patterns and global change effects. Sci Total Environ . 2020 Dec 1;746:140998.
- The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: how far have we come and where do we go from here? Environ Microbiol . 2020 Jun;22(6):2107-2123.
Share
Similar Post:
-
Carrying Capacity: Definition, Graph, and Examples
Continue ReadingCarrying Capacity Definition
The carrying capacity of a biological species in a given habitat, in biology and environmental science, refers to the maximum number of individuals (of that species) that the ecosystem can carry and support, taking into account its geography and physical properties.
What is Carrying Capacity?
In ecology, carrying capacity refers to an environment’s maximum load. The physical characteristics of the surroundings function as restraints (e.g. food, water, competition, etc.). As a result, the population limit is likely to be influenced by these factors. In essence, food availability is a critical element since it influences the size of a species’ population. It does so in such a way that if food demand is not satisfied for a length of time, population size will gradually decline until resources become enough. When food availability surpasses demand, on the other hand, the population will quickly grow and eventually plateau when the source becomes depleted.
The population size at which the population growth rate equals zero is also known as carrying capacity. It should not be confused with the phrase “equilibrium population,” which refers to a population whose gene frequencies have reached a state of balance between mutation and selection pressure.
Carrying capacity refers to the quantity and density of ancient people sustained by a particular location in archaeology. The carrying capacity of an ecosystem is determined by the maximum population during a certain period in this branch of study. However, studies of human history show that the notion of a maximum human population size is extremely uncommon. Human population density varies most of the time, especially as real food production fluctuates for that location or region.
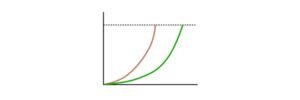
Carrying Capacity Graph
A carrying capacity graph is shown in the image below. The green dotted horizontal line represents the carrying capacity (symbol: K) for a biological species, which describes the number of creatures that the environment can sustainably maintain for a particular time.

It’s worth noting that it’s the same as stable equilibrium, which refers to a population size that has reached a steady-state as it approaches carrying capacity. “Zero-growth” is shown at this point. The growth is shown as an S-shaped curve (a characteristic of logistic growth). When the growth rate is sluggish initially (lag phase) and then accelerates, the S-shape logistic growth emerges (exponential phase). Then, once the population approaches carrying capacity, the pace slows down again.
However, rather than a flat line as represented in the graph, the population tends to rise and dip in oscillations from carrying capacity in the current world.
Carrying Capacity Equation
The equation for the change in population size may be used to derive a formula for the carrying capacity (K):
dN/dt = rN(1-N/K)
The formula for calculating a population change is as follows:
K = rN(1-N)/dN/dt
r denotes the intrinsic rate of growth
N is the population size
dN/dt is the population size change
Carrying Capacity of an Ecosystem
The rate of population growth is constrained by the Earth’s resource availability. A population’s growth rate may be quicker than average, resulting in a J-shaped curve. When the birth rate of a species exceeds its mortality rate, exponential growth occurs. This trend, however, rapidly reverses when resources become scarce. The rate of growth has slowed.
It soon achieves a stable equilibrium, in which the biomass in a particular region seems to remain constant over time. At this stage, the mortality rate within a population appears to be offset by the birth rate. This indicates that the per capita birth and mortality rates are equal.
When deaths appear to outnumber births, however, it implies that the carrying capacity has been reached. It’s an example of an overshoot. It’s possible that the population will go below the carrying capacity. This can happen during illness and parasite epidemics, for example.
The carrying capacity of an ecosystem is influenced by a number of variables. Food supply, water supply, habitat space, intraspecific and interspecific competition, physical variables (e.g. severe heat, drought, etc.), chemical factors (e.g. pH, mineral deficiency, etc.), and anthropogenic influences are all examples of these factors. Environmental resistance refers to the combination of several variables that limit a species’ biotic potential.
Carrying Capacity Examples
i. Turtle Population
When the maximum population size for a specific region with limited resources is achieved, the population of that area may exceed carrying capacity.
For example, a pond with 10 turtles will be sufficient to support the species’ population. The turtles may survive and breed at an exponential rate since there is enough water, food, and room. Competition, on the other hand, becomes more intense as the population rises. Food, water, and space are all in competition for turtles.
Male turtles battle for mates with other males. These variables will restrict the turtles’ biotic potential. When a population appears to be constant, such as 100 turtles, the carrying capacity for that region can be estimated to be 100 turtles.
ii. Forest Population
Another example is a forest’s tree population. Assume that a forest has a carrying capacity of a hundred trees. This means the trees will be able to develop without having to compete for sunshine, nutrients, and space. This implies that the new sprouts may not be able to flourish as well as the older trees, since the taller and older trees will create a shadow over them, making it difficult to reach from below.
Factor Affecting Carrying Capacity
Humans divide the population into sub-populations with distinct demands based on their lifestyle. Some of them, for example, have an omnivorous diet, while others are totally vegan. As a result, the demand for food resources may fluctuate. Humans have also used technology to solve and reduce competition for resources such as space, food, and water.
Agriculture and animal husbandry, for example, contributed to the expansion of the food supply. To meet food demands, humans have learnt to grow crops and raise animals. They ultimately figured out how to construct a secure haven away from predators. Certain contemporary technologies and anthropogenic activities, on the other hand, have a significant negative impact on the population of other species. To develop residences and businesses, some woods and terrestrial ecosystems were destroyed.
During rain and irrigation, pesticides used to fight agricultural pests leach nutrients from the soil. Because of poor garbage disposal, bodies of water have become contaminated.
Many elements in nature restrict population increase. As a result, despite technical advancements that reduce resource rivalry, the human population must contend with additional factors. Sanitation, illnesses, epidemics, and medical treatment are examples of such factors.
Ecological Footprint
The global carrying capacity for humans is predicted to be nine to 10 billion people based on Earth’s demographics and research study statistics. The world’s population is approaching 8 billion people.
The ecological footprint can be utilised as a starting point for research. It is a method of ecological accounting that calculates the human demand on nature. On a global scale, it can assist in determining demand against the planet’s ability to renew. Furthermore, research shows that the Earth has been in an ecological overshoot.
Humans consume more resources and generate trash at a quicker pace than the ecosystem can “heal” or replenish itself. 85 percent of humankind lives in nations with an ecological deficit, meaning their ecological footprint for consumption exceeds their biocapacity.
Carrying Capacity Citations
- Aging Human Populations: Good for Us, Good for the Earth. Trends Ecol Evol . 2018 Nov;33(11):851-862.
- A Quantitative Assessment of Sustainable Development Based on Relative Resource Carrying Capacity in Jiangsu Province of China. Int J Environ Res Public Health . 2018 Dec 9;15(12):2786.
- Carrying Capacity of Spatially Distributed Metapopulations. Trends Ecol Evol . 2021 Feb;36(2):164-173.
Share
Similar Post:
-

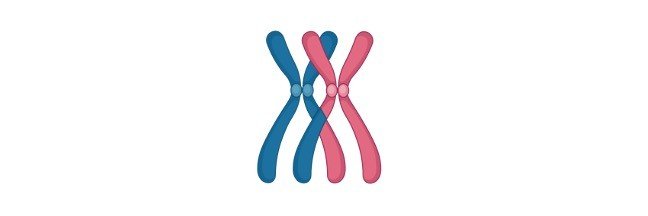
Chromosomes vs Chromatids: Definition and Examples
Continue ReadingChromosomes
Chromosomes are made up of DNA and proteins found in the cytoplasm of the nucleus. In structure they look like thread. Under the light microscope, they can be seen in the metaphase stage, found in the center of the cell. As prokaryotes lacks nucleus, these chromosomes are found in the nucleoid region and are circular in shape. In the plasmid, extrachromosomal DNA might be seen in prokaryotes for the horizontal transfer of gene.

Chromosome and packaging proteins work together in eukaryotes so that chromosome does not end up tangled. As chromosome are huge, they wont fit in the nucleus, thus they are condensed by chromatin fibers to form a structure called chromatin.
In the mitochondria and chloroplast, chromosomes would also be found. In different cell cycle stages the chromosome structure might vary, such as coiled, uncoiled, replicating, dividing and etc. There are a pair of 23 chromosomes, in which one pair are the sex chromosomes called allosomes. The remaining 22 are body chromosomes called autosomes.
All the genetic information about the cell is encoded within the chromosomes. Variation is seen due to crossing over take place while reproduction. Due to mutations, various chromosomal abnormalities would be seen leading to severe conditions.
Chromatids
After the chromosomes get duplicated, it consist of two identical parts called the chromatid. After the uncoiling of chromosome, chromatids are formed. Within the center of the chromatid lies the centromere and are less condensed.

These are called as sister chromatids. When two chromatid exchange the genetic information with the help of chiasmata, it forms non-sister chromatid. Only during the cell division, chromatids are found as later they will again separate to form chromosomes and thus are present for a short period of time.
Although chromatids are homozygous, they can be heterozygous due to mutation. They play a role in meiosis during the prophase for genetic information exchange through chiasmata, thus ensuring variation to be seen. Protein forming is not possible by chromatids.
Chromosomes vs Chromatids
From the chromosome forms the chromatid which are identical and have a temporary role to play. Chromosomes are extremely thin made up of proteins, DNA which contains genetic information. Chromosomes can be single as well as double stranded, whereas chromatids are double stranded.
Chromatids are less condensed and Chromosomes are quite condensed. Chromatids are homozygous whereas, chromosomes are non-identical. In structure chromatids possess a long structure made up of fiber. Chromatids have ribbon like structure. DNA found in chromatid is freely moving, whereas in chromosome is tightly packed.
Chromatids are temporary structures only present during cell division while the chromosomes are present for always. Centromere is found in chromatids, while it is lacking in Chromosomes.
Chromosomes can replicate independently while the Chromatids cannot replicate as well as duplicate. Protein synthesis cannot be carried out by chromatids and is done by chromosome. Functions of chromatids are to keep a check on the cell number after the division has taken place. The transfer of genetic material from one generation to the other is the function of the chromosomes.
Chromosomes vs Chromatids Citations
- Separating sister chromatids. Trends Biochem Sci . 1999 Mar;24(3):98-104.
- Epigenetically distinct sister chromatids and asymmetric generation of tumor initiating cells. Cell Cycle . 2018;17(18):2221-2229.
- Chromosomics: Bridging the Gap between Genomes and Chromosomes. Genes (Basel) . 2019 Aug 20;10(8):627.
Share
Similar Post:
-
Chemical Compound: Definition, Structure, and Examples
Continue ReadingWhat is Chemical Compound?
Chemical compound is the one that is made up of various elements that are bounded with chemical bonds is called as Chemical compound. It is said that all compounds are substances but not every substance is a compound, as pure compounds are also made up of chemical substance. Substance can be defined as those which are made up of various elements having different composition and properties. A substance made from only a single atom is called as a chemical element.
Compound is formed from the element, when elements are linked to each other by a chemical bond. For example, sodium and chlorine come together to form sodium chloride. Thus, in this case it is an allotrope but not a chemical compound. Another example is diamond. Elements such as NaCl, carbon dioxide, water are naturally combined elements. These elements are produced due to the nucleosynthesis such as supernovas nucleosynthesis, stellar nucleosynthesis and others.
Compound Definition
The word compound originates from a Latin word where “com” means together and “ ponere” means to put. The word compound has various meaning in various fields. In zoology it means, various organism when come together to form a colony is called as a compound. Compound also means when two or more elements combine together is called as compound. In botany, when all the parts of a plant come together to form a leaf or a fruit from the ovaries, is called as a compound leaf or a fruit.
Chemical Compound Characteristic
For the formation of a compound, the number of atoms are proposed by the chemical formula. The element is specified with the symbol and the number of atoms is indicated by the subscript. The atoms are binded to each other by the bond between them. These bonds are of 4 types: Hydrogen bond, Ionic bond, Covalent bond and Metallic bond.
The transfer of electrons from one atom to other is called as Ionic bond. Positively charged ions are the cations and the negatively charged ions are called as anions. Between the anions and the cations exists an electrostatic attraction.
Example of ionic compound are NaCl, where Cl is the anion and Na+ is the cation. Base consist of OH– ions, whereas the H+ are the acidic ions. Salt is formed when acid and base reacts with each other. Sharing of electrons between the atoms is called as Covalent bond.
For example in the water molecule, the oxygen atom is shared by two hydrogen atoms, having covalent bond. The bond is of single type when, shared between two electrons. Between two elements 4 electrons being shared is called as double bond. As it is double bonded, one bond is sigma and the other is pi bond. When six electrons are being shared it is called as triple bond, with two pi bond and one sigma bond.
Out of three bond, triple bond is the strongest whereas the single bond is the weakest. In hydrogen bond, a bridge is formed between two atoms, which requires less energy. It is a type of electrostatic bond. In hydrogen bond, a polar hydrogen atom interacts with a polar electronegative atom. In simple words the electronegative atom is attracted by the hydrogen atom.
The DNA and protein secondary and tertiary structure are formed by hydrogen bonds. Although hydrogen bond is weak. Another bond is the metallic bond where, positively charged metal ions and electrons undergo a bonding. Through the metallic bond, intermetallic compounds are formed.
Organic vs Inorganic Compound
Compounds possessing carbon atom is called as Organic compound; whereas the one not containing carbon atoms are called as Inorganic compound. Example of organic compounds are living things. Example lipid, protein, nucleic acid and others. Thus, these compounds when they decompose are broken into smaller fragments. Organic compounds are also secreted by humans, which also is a part of the surrounding environment such as soil. This further serves as a nutrition source to other organism when it reaches water.
Chemical Compound in Biology
When various parts forms material it is called as Compound. From the compound leaf forms the leaflets. Fruits is said to be compound if it is formed from the ovaries.
Chemical Compound Citations
- Chemical compound navigator: a web-based chem-BLAST, chemical taxonomy-based search engine for browsing compounds. Proteins . 2006 Jun 1;63(4):907-17.
- Endocrine disruptor compounds in environment: As a danger for children health. Pediatr Endocrinol Diabetes Metab . 2018;24(2):88-95.
- What is the meaning of ‘A compound is carcinogenic’? Toxicol Rep . 2018 Apr 7;5:504-511.
Share
Similar Post:
-

Cytoplasm: Definition, Function, and Examples
Continue ReadingCytoplasm Definition
All creatures are made up of cells, which are the structural, functional, and biological units. It is a protoplasm-containing membrane-bound structure. Protoplasm is the cell’s fluid life substance. Protoplasm is sometimes used interchangeably with the term cytoplasm. With various sources, the nucleoplasm is mixed in with the protoplasm. Thus, in a tighter sense, protoplasm consists mostly of cytoplasm and nucleoplasm. The protoplasmic contents between the cell membrane and the nuclear envelope make up the cytoplasm.
What is Cytoplasm?
The cytoplasm is the cell’s jelly-like material. It refers to all of a cell’s contents, with the exception of the nucleus in eukaryotic cells. Except for the nucleus, the cytoplasm of a eukaryotic cell consists of the cytosol, vesicles, the cytoskeleton, inclusions, and organelles.
A eukaryotic cell’s cytoplasm is the area of the cell between the cell membrane and the nuclear envelope. A eukaryotic cell’s protoplasm is made up of the cytoplasm and nucleus. The cytoplasm is simply everything contained by the cell membrane in prokaryotic cells that lack a well-defined nucleus. The cytosol and all other cellular components, including the chromosome in the nucleoid region, are therefore included inside it.
The tasks of cell expansion, growth, and metabolism are carried out in the cytoplasm of both eukaryotes and prokaryotes. Cellular organelles are found in the cytoplasm of eukaryotic cells. These organelles serve a specific purpose.
The nucleus, for example, is the organelle that stores genetic material and hence regulates gene expression to govern cellular functions such as metabolism, growth, and reproduction. Chloroplasts are photosynthesis-critical plastids that contain green pigments. Mitochondria are the organelles responsible for generating energy for a variety of metabolic activities.
The endoplasmic reticulum is a network of flattened sacs or tubules that participate in lipid production, carbohydrate metabolism, drug detoxification, and receptor attachment to cell membrane proteins. It also plays a role in intracellular transport, such as transporting rough endoplasmic reticulum products to other cell sections such as the Golgi apparatus.
Membrane-bound stacks make up the Golgi apparatus. It is involved in glycosylation, molecular packing for secretion, lipid transport inside the cell, and the formation of lysosomes. Vacuoles and ribosomes are two more cytoplasmic organelles present in the cytoplasm. Cytoplasmic streaming refers to the flow of cytoplasm surrounding vacuoles in plants.
Protein and RNA make up ribosomes, which are where protein is made. Some ribosomes are unattached to the endoplasmic reticulum, whereas others are. In an intact cell, the cytosol (the fraction of the cytoplasm that remains after the organelles have been removed) is the watery component of the cytoplasm. Water, organic molecules, and dissolved ions make up this mixture.
Water makes up the majority of the cytosol component, accounting for around 70%. Potassium, sodium, chloride, bicarbonate, amino acids in proteins, magnesium, and calcium are the most common ions found in the mammalian cytosol.
The cytosol is the location of numerous chemical processes in the body. It is where the majority of metabolic processes occur in prokaryotes (others occur in the cell membrane). It is where organelles and other cytoplasmic components are hung in eukaryotes.
The cytosol is involved in osmoregulation and cell signalling because it contains dissolved ions. In endocrine, neuron, and muscle cells, it is also involved in the generation of action potentials.
Biochemical Reaction in Cytoplasm
It is where the majority of metabolic processes occur in prokaryotes (others occur in the cell membrane). It is where organelles and other cytoplasmic components are hung in eukaryotes. The cytosol is involved in osmoregulation and cell signalling because it contains dissolved ions. In endocrine, neuron, and muscle cells, it is also involved in the generation of action potentials.
Cytoplasm Function
The cytoplasm is a place where cells develop and metabolise. The cytoplasm produces and degrades a variety of biomolecules. Glycolysis, for example, takes place in the cytosol.
Glycolysis is the first metabolic route of cellular respiration, turning monosaccharides, usually glucose, into pyruvate and producing high-energy biomolecules like ATP in the process. The citric acid cycle and oxidative phosphorylation are two more cellular respiration processes that take place inside the mitochondria.
Cytoplasm Citations
Share
Similar Post:
-

Fermentation: Definition, Process, and Examples
Continue ReadingFermentation Definition
Fermentation is a Latin word which means causing fermentation. Fermentation is a type of metabolic process, in which the organism are responsible for breaking the sugar into alcohol or acid. These processes require chemical energy which is obtained from ATP, to carry out the reaction.
What is Fermentation?
Fermentation can take place in both aerobic in presence of oxygen and anaerobic condition in the absence of oxygen. Degradation of molecules such as sugar produces energy through aerobic and anaerobic respiration. The steps of aerobic respiration are starting from glycolysis where the 6-carbon sugar molecule is broken into a 3-carbon molecule called the pyruvate.
Then acetyl coenzyme A is formed from pyruvate. Further as the acetyl coenzyme A gets broken down to form CO2 from the citric acid cycle. To the NADH and FADH2 the carbon and the hydrogen atoms get transferred. From these two carrier the energy then reaches the electron transport chain from which the energy is obtain for ATP synthesis.
Oxygen is the final electron acceptor which is not required in anaerobic fermentation and gets replaced with sulphate and nitrate molecule. Although people consider fermentation and anaerobic respiration similar but electron transport chain step is absent in fermentation.
The last step is conversion of pyruvate to acetaldehyde. Depending on the fermentation, the byproducts are formed. Lactic acid is formed in lactic acid fermentation. In alcohol fermentation, along with alcohol, CO2 is also formed.
In prokaryotes and as well as in eukaryotes, fermentation takes place. When the amount of oxygen is very minimal, fermentation is used to obtain energy. For example, when we perform exercise ATP is generated from muscle cell to provide energy through aerobic respiration. However, when ATP supply competes with the oxygen supply, muscle cell carries out lactic acid fermentation to obtain energy in limited oxygen content. Once the level is restored, aerobic respiration again begins.
To synthesize ATP, fermentation is carried out by obligate anaerobes. An example is Neocallimastix. However, there are organism that will undergo fermentation even when the oxygen is present are called as facultative anaerobes. Example are yeast, Kluyveromyces lactis and baker’s yeast are few examples.
In commercial industries, to produce wine and other alcohol, yeast is used to carry out fermentation, whereas in dairy industry bacteria and fungi are used.
Importance of Fermentation
Due to the breakdown of sugar, chemical energy is produced, that is an advantage to the anerobic organism in the anerobic environment such as hydrothermal vent, soil and mud. These type of organism are very vital as they obtain energy by fermenting molecule and excrete the by-product directly into the environment, which can be used other organism or enter the nutrient cycle.
There are various organism residing in other living organism such as various microbiota in the human gut. Within cattle as well, organism are present that cannot breakdown their food, thus reside within the cattle to obtain it and in return have enzymes which aid in digesting starch and cellulose. Similarly, even humans have such organism present, these flora get shelter and food and in return they synthesize vitamins required.
Although these microflora reside within human and other living organism. They can also be opportunistic if found at the wrong position within the humans and animals.
When the oxygen content is limited and we are performing strenuous activities then body has to meet up the need and thus, has lactic acid fermentation to generate ATP. Through aerobic respiration, using one glucose molecule 38 ATPs are formed, whereas through fermentation only two molecules are formed. Thus, aerobic respiration is a lengthier process. Only when quick energy is required, fermentation is an alternative.
As the red blood cells mature, they no longer contain mitochondria. Thus, fermentation is carried out by such cells which do not contain mitochondria. Electron transport chain redox reaction and citric acid cycle occurs in the mitochondria. Thus, glycolysis takes place where electrons are transferred and this happens in the cytosol. So that the mature RBCs do not use the oxygen they transport, they obtain energy from lactic acid fermentation.
To make wine, cheese, bread, beverages, soy sauce and others fermentation is important. For example, for wine production, to the grapes the yeast is added for fermentation to occur. In case of making of bread, dough is prepared and allow to ferment after the yeast is added which will secrete CO2 and the bread will rise. Similarly, bacteria undergoes fermentation with milk to form cheese.
Fermentation Process
There are several steps in fermentation and they are: Glycolysis which means breaking of sugar molecule. The 6-carbon sugar molecule breaks into 3 carbon compound pyruvates, which uses chemical energy such as ATP , thus called the energy investment step. In the next step, ATP is produced by substrate level phosphorylation, along with NADH. In the electron transfer step, pyruvate is used, which gets NAD+ and is again reobtained as it was lost in the first step of glycolysis. After glycolysis citric acid cycle does not take place, thus only 2 ATP molecules are gained. This process takes place in the cytosol.
Types of Fermentation
There are 3 types of fermentation, ethanol fermentation, lactic acid fermentation and acetic acid fermentation. In both eukaryotes and prokaryotes, fermentation takes place. The most commercial fermentation are bacterial and yeast fermentation.
i. Ethanol Fermentation
In this fermentation, the product obtained is ethanol. The steps involved are glucose molecule forms two pyruvate molecules in the glycolysis step. Then it further forms acetaldehyde and excretes CO2. From the NADH, a hydrogen ion is released and combines with acetaldehyde to form alcohol particularly ethanol, with NAD+ left. Pyruvate carboxylase and alcohol dehydrogenase are the enzyme involved in the second and third step.
Anaerobic bacteria such as yeast can also produce ethanol. Example Saccharomyces cerevisiae. They have been used in commercial industry to make alcohol, bread and others. In anaerobic conditions, fishes also produce ethanol in their myotomal muscles. Lactic acid fermentation is also possible.
ii. Lactic Acid Fermentation
It takes place in the cells cytosol and sugars are converted to lactate. Lactic acid fermentation is of two types: Homolactic and Heterolactic fermentation. End-product is lactate in homolactic fermentation. In Heterolactic fermentation, there are other end products as well such as carbon dioxide.
Homolactic fermentation also begins with glycolysis, and pyruvate is directly reduced by NADH, thus forming lactate and obtains NAD+. Lactate dehydrogenase is the enzyme carrying out the reaction.
While performing strenuous activities, there is a waste product which is released from the muscle to the blood to the liver, where it will get converted to pyruvate. This is known as Cori cycle and the enzyme is lactate dehydrogenase. It is a bidirectional reaction.
Fermentation Equation: The equation for ethanol fermentation is:
C6H12O6 → 2C2H5OH+ 2CO2 + Energy
As the 6-carbon sugar molecule is broken into two 2 pyruvate molecules, thus there are two molecules of ethanol. The byproducts is carbon dioxide. There is a gain of two ATP molecules.
iii. Lactic Acid Fermentation
The equation for homolactic fermentation of lactic acid is:
C6H12O6 → 2 CH3CHOHCOO– + Energy
As the 6-carbon sugar molecule is broken into two 2 pyruvate molecules, thus there are two molecules of lactate. There is a gain of two ATP molecules.
Lactate can be metabolized further by bacteria such as Leuconostoc mesenteroides, thus having by-products such as carbon, dioxide and etc.
C6H12O6 → CH3CHOHCOO– + C2H5OH + CO2 + Energy
A heterolactic acid fermentation involves few byproducts as well. 1 ATP is gained in this fermentation.
Fermentation Products
Depending on the fermentation and the enzymes involved, the products will vary. For example, from pyruvate, ethanol is produced with the enzyme pyruvate carboxylase and alcohol dehydrogenase. To product lactate, pyruvate is required and the enzyme is lactate dehydrogenase. Carbon dioxide, energy, acetate, hydrogen gas are the by-products.
For the formation of acetic acid, bacteria will break down sugar to form acetic acid. example is vinegar. When acetic acid bacteria acts on sugars it results in the formation of vinegar.
CH3CH20H + O2 → CH3COOH + H20
Here ethanol reacts with water to form acetic acid, which is an oxidation and fermentation process. If fermentation requires light it is called as photo-fermentation and in the absence of light it is called as dark fermentation.
History of Fermentation
Since ancient times, fermentation has been carried out. Examples are making wine from grapes, octli from agave, from malted barley making beer and from maize chicha. At that time, they used wooden containers to store these beverages in them, but how they were aware of this concept is not well known. However, in 17th century people as people became aware about lenses and microscope.
All these microorganism could be seen microscope to open up the world. Through microscope it became clear that the microorganism associated with fermentation is yeast and they divide by budding in the fermentation process.
However, Louis Pasteur, was showed that yeast are living organism and transform sugar into alcohol. They could also carry out in the absence of oxygen. Alcoholic fermentation was termed by Louis Pasteur, where he said that the change in the sourness is due to the presence of living organism, thus converting into desired product.
In 19th century, a German chemist pulverized the yeast cell, to make alcohol from sucrose. The one responsible for catalyzing the reaction was termed as “zymase”. Further the research has kept on growing and many more organism have been discovered.
Fermentation Citations
- The Application of Fermentation Technology in Traditional Chinese Medicine: A Review. Am J Chin Med . 2020;48(4):899-921
- Why, when, and how did yeast evolve alcoholic fermentation?FEMS Yeast Res . 2014 Sep;14(6):826-32.
- Fermentation, fermented foods and lactose intolerance. Eur J Clin Nutr . 2002 Dec;56 Suppl 4:S50-5.
Share
Similar Post: