What is Cell Culture?
Cell culture refers to the removal of cells from an animal or plant and their subsequent growth in a favourable artificial environment.
The cells may be removed from the tissue directly and disaggregated by enzymatic or mechanical means before cultivation, or they may be derived from a cell line or cell strain that has already been established.
Mammalian cell culture is used widely in academic, medical and industrial settings.
It allows the study of physiology and biochemistry of the cell and its development.
It has also widen the scope and its application in the field of cell and molecular biology where the use of reproducible model systems is attained by cultured cell lines.
For medical use, cell culture provides test systems to assess the efficacy and toxicology of potential new drugs.
Large- scale mammalian cell culture has allowed production of biologically active proteins, initially production of vaccines and then recombinant proteins and monoclonal antibodies; recent innovative uses of cell culture include tissue engineering to generate tissue substitutes.
Basic Requirements for Successful Cell Culture
The first necessity is a well-established and properly equipped cell culture facility.
The level of bio containment required (Levels 1–4) is dependent on the type of cells cultured and the risk that these cells might contain, and transmit, infectious agents.
All facilities should be equipped with the following: a certified biological safety cabinet a centrifuge, preferably capable of refrigeration and equipped with appropriate containment holders that is dedicated for cell culture use; a microscope for examination of cell cultures and for counting cells; and a humidified incubator set at 37°C with 5% CO2 in air.
The second requirement for successful cell culture is the practice of sterile technique prior to beginning any work, the biological safety cabinet should be turned on and allowed to run for at least 15 minute to purge the contaminated air.
All work surfaces within the cabinet should be decontaminated with an appropriate solution; 70% ethanol or isopropanol are routinely used for this purpose.
A third necessity for successful cell culture is appropriate, quality controlled sterile reagents, culture media and required sterile plastic wares.
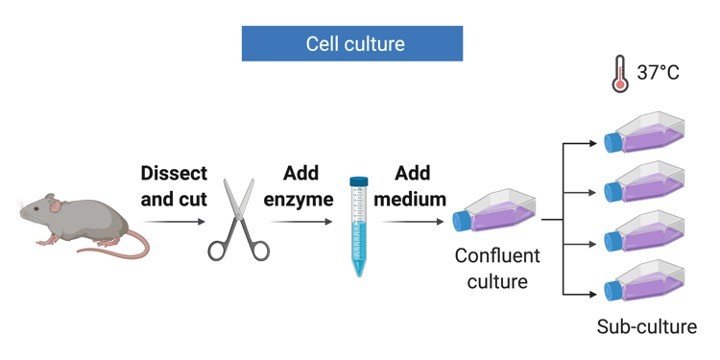
Adopted from BioRender
Types of Cell Culture
There are two types of cell culture system, Primary and continuous culture.
1. Primary Culture: Primary cultures are derived directly from excised, normal animal tissue and cultures either as an explant culture or following dissociation into a single cell suspension by enzyme digestion.
The preparation of primary cultures is labour intensive and they can be maintained in vitro only for a limited period of time.
During their relatively limited lifespan primary cells usually retain many of the differentiated characteristics of the cell in vivo.
2. Continuous Culture: Continuous cultures are comprised of a single cell type that can be serially propagated in culture either for a limited number of cell divisions (approximately thirty) or otherwise indefinitely.
Cell lines of a finite life are usually diploid and maintain some degree of differentiation.
The fact that such cell lines senesce after approximately thirty cycles of division means it is essential to establish a system of Master and Working banks in order to maintain such lines for long periods.
Continuous cell lines that can be propagated indefinitely generally have this ability because they have been transformed into tumour cells.
Tumour cell lines are often derived from actual clinical tumours, but transformation may also be induced using viral oncogenes or by chemical treatments.
Transformed cell lines present the advantage of almost limitless availability, but the disadvantage of having retained very little of the original in vivo characteristics.
Cell Culture Morphology
In terms of growth mode cell cultures take one of two forms, growing either in suspension (as single cells or small free floating clumps) or as a monolayer that is attached to the tissue culture flask.
The form taken by a cell line reflects the tissue from which it was derived e.g. cell lines derived from blood (leukaemia, lymphoma) tend to grow in suspension whereas cells derived from solid tissue (lungs, kidney) tend to grow as monolayers.
Attached cell lines can be classified as endothelial, epithelial, neuronal or fibroblasts and their morphology reflects the area within the tissue of origin.
There are some instances when cell cultures may grow as semi-adherent cells, ( e.g. marmoset B-lymphoblastoid cell line), where there appears to be a mixed population of attached and suspension cells.
For these cell lines it is essential that both cell types are subcultured to maintain the heterogeneous nature of the culture.
In vitro age of cell culture Two terms are predominantly used to define the age of a cell culture:
1. Passage number indicates the number of times the cell line has been sub-cultured.
2. The population doubling (pd) number indicates the number of cell generations the cell line has undergone i.e. the number of times the cell population has doubled.
Cell Culture Maintenance
In culturing mammalian cells in vitro, one attempts to reproduce in a culture vessel the physiological environment and characteristic responses of individual cell types.
At a minimum, the fluid medium in which cells are cultured must provide for their nutritional requirements, provide an energy source, maintain pH, and provide a level of osmolarity compatible with cell viability.
Culture media commonly used today consist of two parts: a basal nutrient medium and supplements.
The basal nutrient medium, such as Dulbecco’s modified Eagle’s medium (DMEM; also known as Dulbecco’s minimal Eagle’s medium), RPMI 1640, or Ham’s F-12, is a buffered aqueous solution of inorganic salts, vitamins, amino acids and other anabolic precursors, energy sources such as glucose and glutamine, and trace elements.
Supplements are either undefined, such as fetal bovine serum (FBS), tissue extracts, and conditioned medium, or defined, such as hormones and growth factors, transport proteins, and attachment factors.
The compositions of basal nutrient media and medium supplements may vary considerably; however, both components of the complete medium are necessary for support of cell viability and proliferation.
There are two formats of media available i.e. dehydrated and liquid media.
Preparation of Cell Culture Media
Requirements:
1. Dehydrated medium (i.e. RPMI or DMEM)
2. Double autoclaved water
3. Autoclaved bottles for storing medium
4. 0.22 micron filtration unit (capacity 1 litre)
5. Antibiotics (Penicillin, Streptomycin, Amphotericin B)
6. Additives for the medium as required (like sodium bicarbonate, sodium pyruvate, glutamate etc.)
7. 1N NaOH and HEPES
8. Conical Flask (1L or 2L for dissolving the dehydrated medium)
9. Measuring Cylinder
10. Discard beaker
Cell Culture Procedure
1. In a sterile biosafety cabinet dissolve powdered medium with constant stirring in a 0.8× to 0.9 × volume of water. (If a commercially prepared liquid medium is being used, add penicillin and streptomycin from commercial stock solutions and proceed to step 8)
2. Add an amount of HEPES that yields a concentration of 15 mM in the final volume of medium. (Omit this step if the powdered medium is formulated with HEPES.)
3. Add the amount of sodium bicarbonate recommended by the medium supplier for use in a CO2-controlled atmosphere (e.g., 14 to 36 mM in 5% CO2 atmosphere). (Omit this step if the powdered medium contains sodium bicarbonate.)
4. Add glutamine to give a final concentration of 2 mM and pyruvic acid to give a final concentration of 0.01% (w/v). (Omit this step if the powdered medium contains glutamine & pyruvic acid.)
5. Add penicillin G to give a final concentration of 100 IU/ml and streptomycin to give a final concentration of 50 μg/ml.
6. (Other antibacterial agents or antifungal agents should not be routinely included in culture medium. Gentamicin at a final concentration of 50 μg/ml or kanamycin at 100 μg/ml may be useful in eliminating gram-positive and gram-negative bacteria from primary cultures or from irreplaceable cultures, but it is best to discard any cultures that are contaminated with bacteria, yeast, or fungi.)
7. Adjust the pH of the medium to 7.4 with 1 N NaOH, and add water to achieve the final (1×) volume. Readjust the pH of the medium to 7.4, if necessary.
8. Sterilize the medium by filtration through a 0.2-μm filter. Store the medium at 4°C in the dark. Vacuum-operated filtering units or bottle-top filters are useful for small volumes of medium (0.1 to 2 liters), whereas filter capsules (2 to 5 liters) or filter stands (>10 liters) that are used under positive pressure are more suitable for larger volumes.
9. Make an aliquote of prepared media and keep at 37°C to check for any contamination.
10. Add serum (5-20%) to the desired final concentration at the time of use.
Cell Culture Precautions
Basal nutrient medium and the serum supplement should be stored individually at 4°C, and the complete medium should be made up at the time of use and only in the volume necessary.
Working volumes of serum should be stored at 4°C and used within several weeks.
Serum should not be subjected to repeated freezing and thawing, but it can be stored for at least 2 years at −20°C with little deterioration in growth-promoting activity.
In this way, medium components are not wasted, and the chances of detecting, isolating and eliminating contamination with minimal losses are increased.
Cell Culture Citations:
Share









