Category: Biology
-

Red Algae: Definition, Types, & Examples
Continue ReadingWhat is Red Algae?
The phylum Rhodophyta includes red algae, which is derived from the Greek words rhodon which means “rose” and phyton which means “plant.” They have a red in colour due to the presence of accessory pigments such as phycoerythrobilin, phycocyaniobilin, phycourobilin, and phycobiliviolin in phycobillisomes, as well as the green pigment chlorophyll pigments (often, chlorophyll a).
They save their carbohydrate reserves in the form of Florida starch. They are thought to be among the first eukaryotic algae. With almost 7,000 species identified, the group could be the largest. Over 90% of red algal species are found in marine settings, with the remaining 10% found in freshwater and two species located in coastal caves. Rhodella, Compsopogon, Stylonema, Bangia, Porphyra, Porphyridium cruentum, Hildenbrandia, Nemalion, Corallina officinalis, Ahnfeltia, Gelidium, and other red algae species this are examples.
Red Algae Classification
Protista is one of the taxonomic kingdoms in the previous categorization framework, known as the five kingdom scheme. It’s made up of protozoa that look like animals, algae that look like plants, and slime moulds and water moulds that look like fungi. Protista is separated into multiple phyla as a result.
Euglenophyta, Chrysophyta (diatoms), Pyrrophyta (dinoflagellates), Chlorophyta, Phaeophyta, and Rhodophyta are the phyla that divide the plant-like or algal species. Recent research and findings, on the other hand, may lead to modifications in taxonomic ranks and the development of new classification systems.
Red Algae Characteristic
The presence of phycobilin accessory pigments such as phycoerythrobilin, phycocyanobilin, phycourobilin, and phycobiliviolin concentrated inside the phycobilisomes causes red algae to appear crimson or red in colour, as their name implies.
Chlorophyll a and d, – and -carotene, lutein, and zeaxanthin are among the pigments found. Floridian starch, a 1-4 branched glucose polymer spread throughout the cytoplasm, represents their carbohydrate reserve. The outside layer of their cell wall is made up of agarose and agaropectin, whereas the interior layer is mostly made up of cellulose.
The absence of flagella and centrioles is another distinguishing trait of red algae. They’re also used to make pit connectors and plugs. Following mitosis, the pit connections and pit plugs form during cytokinesis.
Red algae are the only ones with these structures. Cell-to-cell communication and/or symplastic transport are hypothesised to be aided by pit connections. Red algae can reproduce in two ways: sexually and asexually. The merger of gametes is the method of sexual reproduction. The male gamete, on the other hand, is not motile because it lacks a flagellum. It must be carried to the female gamete by a water stream.
Asexual reproduction is usually accomplished through the formation of spores, fragments, and propagules. Similar to other algal groupings, there is a generational cycle. Red algae, on the other hand, goes through three generations in a row. Two sporophyte generations may follow the gametophyte generation.
The first sporophyte is known as the carposporophyte (due to the production of carpospores), and the second sporophyte is known as the tetrasoporophyte (because to the production of tetrasopores). In a nutshell, the life cycle begins with the creation of male and female gametes in the gametophyte.
The merger of two gametes at the end of this phase results in the development of a diploid zygote. The zygote matures into a carposporophyte, which generates carpospores. The carpospore becomes a tetrasporophyte, which then generates spore tetrads. Tetrads become gametophytes when they germinate.
Tetrasporophyte generation can be skipped in some cases when the carposporophyte produces carpospores that germinate directly into thalloid gametophytes. From single-celled to multicellular, the shapes are varied. Many of them are marine species that can be found along the tropical, temperate, and cold-water coasts and continental shelf zones.
Red Algae Evolution
According to the endosymbiotic theory, a photosynthetic prokaryote was absorbed by an early eukaryotic phagotroph. The symbiosis between the two primitive living forms resulted in the prokaryote becoming an organelle within the eukaryotic cell, specifically a plastid. This event is thought to have triggered the evolution of autotrophic clades such as green algae, red algae, and glaucophytes. However, the red algae are thought to have been implicated in two endobiosis outbreaks.
A secondary event between an ancestral red alga and a eukaryotic heterotroph may have resulted in the diversification of algal species, resulting in the emergence of new clades such as Cryptophyta, Haptophyta, Alveolata, and the heterokonts.
Biological Importance of Red Algae
The ecosystem’s limestone-reef builders are red algae. A good example is coralline algae. They secrete calcium carbonate, which aids in the formation of coral reefs. Nori (Porphyra) and dulse (Palmaria palmata) are two species that are used in Asian and European cuisines, respectively. Some red algae (e.g. Gracilaria, Gelidium, Porphyra, Palmaria, and Euchema) are collected for the manufacture of agar and/or carrageenan products due to the cell wall component.
Red Algae Citations
Antitumor potential of carrageenans from marine red algae. Carbohydr Polym . 2020 Oct 15;246:116568.
Lectins from red algae and their biomedical potential. J Appl Phycol . 2018;30(3):1833-1858.
Red Algae-Derived Carrageenan Coatings for Marine Antifouling Applications. Biomacromolecules . 2020 Dec 14;21(12):5086-5092.
Share
Similar Post:
-

Flying Fox: Description, Habitat, & Facts
Continue ReadingFlying Fox Definition
Kingdom Animalia Phylum Chordata Class Mammalia Order Chiroptera Family Pteropodidae Genus Pteropus Species Over 60 Length Various Weight 0.26 to 3.53 lb (120 to 1,600 grams) Lifespan Up to 15 years in the wild, 30 years in captivity Social Structure Social, live in large colonies Status Various – some are threatened due to overhunting Habitat Tropical Forests Average litter size 1 Food Fruits, vegetation, and insects Predators Snakes, birds of prey, and humans What is Flying Fox?
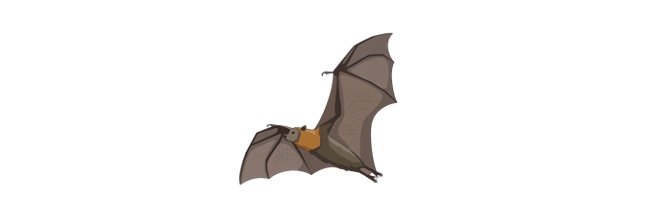
The Fox bat, often known as the Flying Fox, is a megabat genus (Pteropus). This genus has around 60 species of bats that can be found on tropical islands ranging from Madagascar to Australia, Indonesia, and mainland Asia. They’re fruit bats from the past. Flying foxes are the largest bats, with a wingspan of 5 feet (1.5 meters) and a head and body length of 16 inches (40 cm).
The flying fox features a fox- like head with short ears and large eyes, as their name suggests. Their claws are sharp and curled on their toes. These huge bats eat fruit and other plants, as well as insects that they find using their excellent sense of smell. Most species are nocturnal and rely on their vision to travel because they lack the ability to use echolocation like other bats.
These bats have binocular eyesight and are able to see in low light. Individuals and family groupings reside in vast colonies where these sophisticated animals live. They set up permanent and semi-permanent camps in locations where food is plentiful.
These flying foxes can be heard making numerous cries to communicate as they leave to feed or return to sleep at dawn and twilight. They roost on trees throughout the day because they are usually nocturnal. Flying foxes live for a long time and reproduce slowly, with most females having only one offspring each year.
As a result, they are subject to hazards including culling, overhunting, and natural calamities. Overhunting has resulted in the extinction of six species in recent years. Farmers typically regard flying foxes as pests because of the harm they bring to crops.
Flying foxes have been blamed in various nations for ruining fruit and nut crops, including areca in India, almonds, mangos, and guavas in the Maldives, lychee in Mauritius, and stone fruits in Australia. By 2018, the IUCN had assessed 62 species of flying fox.
Three are extremely endangered, seven are endangered, twenty are vulnerable, six are near threatened, and fourteen are of low concern. Eight of the remaining 12 species have insufficient evidence to adequately assess their state, and four are thought to be extinct.
Flying Fox Species
The Grey-headed Flying Fox (Pteropus poliocephalus) is Australia’s largest bat. It is Australia’s sole endemic flying fox species, and the IUCN has classified it as vulnerable.
One of the largest bat species is the Large Flying Fox (Pteropus vampyrus). The males have stiffer and thicker coats than the females, with long and woolly hair. The coat’s colour and texture can also differ between sexes and age groups.
South Central Asia is home to the Indian Flying Fox (Pteropus giganteus). These flying foxes are found in tropical forests and wetlands, and they prefer to sleep among banyan, tamarind, and fig trees near water sources.
Pteropus lylei (Lyle’s Flying Fox) can be found in Vietnam, Cambodia, and Thailand. They can also be found in China’s Yunnan province. They are a medium-sized bat with a black colour scheme and an orange fur collar. These bats are categorised as fragile because they are threatened by habitat loss, hunting, and farm persecution.
The Pteropus scapulatus (Little Red Flying Fox) is a small flying fox that can fly and climb well. They are nomadic bats that wander from one forest to the next or from one coastal location to the next in search of their favoured food. They can be found in the northern and eastern parts of Australia.
Fun Facts About Flying Fox
Flying foxes are intriguing species that live in tropical forests and contribute much to the ecosystem. These winged animals’ biological traits make them particularly interesting to study. Let’s take a closer look at it now!
i. Rapid Digestion
Like other bats, flying foxes have a fast digesting system. They have outstanding chewing and fragmentation abilities. This means that the digestive enzyme has a larger surface area to work with. They have such a fast digestive system that they can start defecating within 30 to 60 minutes of eating. This can help them carry less weight throughout their flight. These bats can eat anywhere from 25 to 35 percent of their body weight every day.
ii. Flying Foxes Carrying Viruses
Hendra virus and Australian Bat Lyssavirus are reported to be carried by flying foxes. These bats are known to carry the Hendra virus (HeV), which can occasionally spread to other species, including horses, causing them to die. HeV was first found in 1994 in samples taken during a respiratory and neurologic sickness outbreak in horses and humans in Hendra, a Brisbane suburb.
Human infection is extremely rare; only seven cases have been documented between 1994 and 2013. Bats can transmit the Australian bat lyssavirus (ABLV) to humans. This virus, which is closely linked to the rabies virus, was originally discovered in 1996.
It has been discovered in four different species of flying fox. Infection with the ABLV virus in humans can cause paralysis, delirium, convulsions, and death.
iii. Vital Role in Ecosystem
Flying foxes play a crucial role in maintaining the health of tropical forests by distributing seeds and pollinating flowers. As they crawl or fly between flowers and trees, pollen attaches to their fur, which they subsequently carry to other plants.
Bats are essential to the ecosystems in which they live, accounting for about half of the animal species present in most tropical forests. These bats are far more important to the environment than previously believed. When it was previously assumed that flying foxes were harmful and caused damage to this crop, researchers have revealed that they are quite successful at pollination durian trees.
The tropical durian fruit is highly regarded in Thailand and Malaysia, where it earns millions of dollars in local and international trade.
Flying Fox Citations
- Hendra virus ecology and transmission. Curr Opin Virol . 2016 Feb;16:120-125.
- Seasonal reproduction in flying foxes, reviewed in the context of other tropical mammals. Reprod Fertil Dev . 1993;5(5):499-521.
- Newly discovered viruses of flying foxes. Vet Microbiol . 1999 Aug 16;68(1-2):83-7.
Share
Similar Post:
-

Galactose: Definition, Function, and Examples
Continue ReadingGalactose Definition
Galactose is one of the three most common monosaccharides, with glucose and fructose as the other two. The most basic form of carbohydrate is monosaccharide.
Simple sugars are distinguished from more complex sugars such as oligosaccharides and polysaccharides. Glycosidic bonds allow monosaccharides to join to generate complex carbohydrates (glycosidic linkages).
What is Galactose?
Hexose monosaccharide galactose is a hexose monosaccharide. It’s a natural substance. C6H12O6 is its chemical formula in general. Galactose has a molar mass of 180.156 g/mol. The melting point is between 168 and 170 degrees Celsius. It has a crystalline structure, is water soluble, and has a sweet flavour.
Galactose vs Glucose vs Fructose
The three most frequent natural monosaccharides are glucose, galactose, and fructose. Glucose, however, is the most prevalent. C6H12O6 is the chemical formula for all three. Because of the six carbon atoms, they are classified as a hexose monosaccharide.
Fructose is a ketose, whereas glucose and galactose are both aldoses. As a result, glucose and galactose have a more physically similar structure. Despite this, the orientation of the hydroxyl group (OH) at carbon 4 can be used to distinguish glucose from galactose structurally.
Galactose also has a greater melting point than glucose. It has a melting point of 168–170 °C, compared to 146 °C for glucose. Fructose, on the other hand, has the lowest melting point of the three (103 °C). Galactose, unlike glucose, is rarely found in a free state. It is frequently found as part of larger macromolecules.
Galactose and glucose, for example, combine to make lactose (milk sugar), a disaccharide. Because glucose is more easily available than galactose or fructose, it is used more frequently in energy metabolism.
Galactose enters glycolysis when there isn’t enough glucose, but it must first be transformed into glucose 6-phosphate before proceeding to glycolysis.
Fructolysis (fructose catabolism) entails fructose phosphorylation by fructokinase to produce fructose 1-phosphate, which is then cleaved into two trioses by aldolase B: dihydroxyacetone phosphate and glyceraldehyde.
Galactose Types
Dextrogalactose (D-galactose) and Levogalactose are the two enantiomers of galactose (L-galalactose). When the glucose stereoisomer rotates plane polarised light clockwise, this nomenclature (based on Fischer projection) identifies D–.
When it rotates plane polarised light in a counterclockwise direction, it is called L–. Hydrolysis produces the dextrotatory form of galactose from milk sugar. Sugar beets, seaweeds, and nerve cell membranes all contain D-galactose. Mucilages provide it with its levorotatory form.
Galactose Metabolism
A monosaccharide, such as galactose, bonds to another monosaccharide through dehydration synthesis, which results in the release of water and the formation of a glycosidic bond. A disaccharide is formed when two monosaccharide units are joined together, but an oligosaccharide is formed when three to ten monosaccharide units are joined together.
Multiple monosaccharides are joined together to form polysaccharides. Galactose unites with another monosaccharide to generate a disaccharide in this case. Lactose, for example, is created when galactose and glucose molecules are combined.
Lactulose, a man-made disaccharide made comprised of galactose and fructose, is another option. A galactan is a polysaccharide made up of repeated galactose units in terms of polymers.
Saccharification is the process of breaking down complex carbohydrates into simpler sugars like glucose and galactose. It entails the process of hydrolysis. This involves an enzymatic action in humans and other higher animals. Lactase, a β-galactosidase enzyme, aids digestion in a diet containing galactose (e.g. lactose in dairy products).
Lactase catalyses the hydrolysis of lactose in the small intestine and breaks the -glycosidic bond, releasing glucose and galactose. In the case of ceramide-rich foods, the lactase β -glycosyleramidase complex releases galactose by breaking the β-glycosidic link in glycolipids. Lactose intolerance is caused by the lack or deficiency of lactase, which prevents lactose from being digested into simpler monosaccharides.
Lactose that is not digested in the small intestine travels to the colon, where it is fermented into lactic acid by gut bacteria. Methane and hydrogen gas are created as a result, causing discomfort, intestinal distention, and flatulence. Water is pulled into the colon by the osmotically active lactic acid, causing diarrhoea.
Lactose can be metabolised by microorganisms such as E. coli, which produces β-galactosidase from its lac operon system.
Galactose is absorbed by intestinal cells (enterocytes) via a sodium-dependent glucose transporter, which is similar to the ATP-driven transport mechanism that absorbs glucose. As a result, during intestinal absorption, glucose competes with galactose. Galactose leaves the intestinal cells and enters the circulation via glucose transporter-mediated transport (Glut-).
A two-phase procedure is used to convert galactose to glucose. The enzyme mutarotase converts β -D-galactose into α-D-galactose in the first phase. -D-galactose is transformed to uridine diphosphate (UDP)-glucose in the final step. The Leloir pathway is frequently used during the last phase.
α-D-galactose is phosphorylated by galactokinase to create galactose 1-phosphate in this route. The uridine monophosphate (UMP) group is then added to galactose 1-phosphate by the enzyme galactose-1-phosphate uridyltransferase, resulting in UDP-galactose.
The enzyme UDP galactose-4′-epimerase then interconverts UDP-galactose to UDP-glucose. The De Ley Duodoroff pathway is an alternative to the Leloir pathway in humans and other species. Galactose that has been transformed to glucose is one method galactose enters the glycolytic pathway. As a result, the entire reaction would be as follows:
Galactose + ATP → Glucose-1-phosphate + ADP + H+
The isomerization of glucose 1-phosphate to glucose 6-phosphate is catalysed by phosphoglucomutase. Galactose metabolism takes place in the liver in humans.
Some glucose molecules are converted to galactose in humans and other mammals so that there is more galactose to mix with glucose to generate lactose. This is especially crucial during the milking process.
Lactose is secreted as milk by the mammary gland, especially during breastfeeding. Note that galactose can also be acquired through dietary sources. Hexoneogenesis is the de novo production of glucose and galactose in the mammary gland.
Galactan is a galactose polymer found in hemicelluloses. Galactose monomers bind together to generate galactans in plants like axlewood ( Anogeissus latifolia) and acacia trees.
Glycosylation is the process of adding a carbohydrate component to proteins and lipids, such as galactose. Galactose is a sugar that is found in a variety of glycolipids and glycoproteins. It could, for example, be a component of cerebroside (a glycolipid comprised of a carbohydrate and a sphingolipid). Glucocerebrosides and galactocerebrosides have glucose and galactose carbohydrate residues, respectively.
Galactose Disorders
Galactosemia is a disease caused by improper galactose metabolism. It’s a rare metabolic condition. A heritable genetic mutation influencing the manufacturing of a Leloir pathway enzyme, galactose 1-phosphate uridyl transferase, is one of the most common causes. Galactosemics should avoid eating a diet high in galactose (and lactose). It could cause diarrhoea, vomiting, and finally cirrhosis if not treated.
Galactose Function
Galactose is a type of monosaccharide that has a variety of biological functions. It can be used as a substitute for glucose when the latter is insufficient to meet an organism’s metabolic needs. It has the ability to enter glycolysis and produce energy. It must, however, go through several preliminary stages before entering the glycolytic pathway.
Galactose is a component of lactose, a milk disaccharide. Lactose is biosynthesized by humans and other milk-producing animals from galactose and glucose.
Milk is an essential source of nutrition, particularly for newborns. Because galactose is a component of cerebrosides, they are referred to as galactocerebrosides rather than glucocerebrosides, which contain glucose rather than galactose.
Galactocerebrosides are abundant in neural tissues and are the primary glycosphingolipid in the brain, which is why galactose is known as the brain sugar. Sulfatide is galactose that has been sulfated afterwards.
Immune response and neurological system signalling are also influenced by sulfatides. Galactose can be found in a variety of plants, including flaxseed mucilages and sugar beet.
A galactose polymer found in the hemicellulose of plants such as axlewood ( Anogeissus latifolia) and acacia trees is known as galactan.
Galactose Citations
- Galactose-Induced Skin Aging: The Role of Oxidative Stress. Oxid Med Cell Longev . 2020 Jun 17;2020:7145656.
- D-Galactose-induced accelerated aging model: an overview. Biogerontology . 2019 Dec;20(6):763-782.
- Galactose metabolism and health. Curr Opin Clin Nutr Metab Care . 2015 Jul;18(4):422-7.
Share
Similar Post:
-

Golgi Apparatus: Definition, Function, & Examples
Continue ReadingGolgi Apparatus Definition
Golgi Apparatus is involved in the packing of proteins and other molecules for secretion, modifying proteins through processes like glycosylation. These membrane-bound stacks are responsible for the production of lysosomes.
What is Golgi Apparatus?
The Golgi apparatus was discovered by an Italian physician Camilo Golgi in 1898. It is a part of the GERL complex and is present in eukaryotic organisms. It is also known as dictyosomes in plants and as the parabasal body in flagellate protozoa. All the Golgi apparatus of a cell is together termed as Golgi complex.

The membranous stacks of Golgi are cisternae that have their origin from the Endoplasmic Reticulum in form of vesicles. The stacks of cisternae in the case of mammals range from 40 to 100. The flattened and fused cisternae comprise stacks that often have microtubule connections in between them.
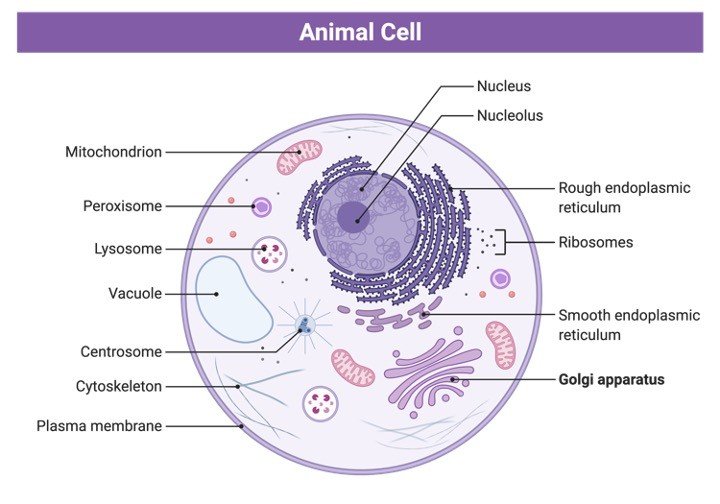
If these interconnecting tubules are removed then now the organelles consist of only individual disks. In plants, the microtubules in stacks are replaced by actin and its location in a cell varies.

It is found proximal to the nucleus especially in the centrosomal region in mammals. Unlike mammals, plants, are not concentrated in any specific region. The usual location of Golgi is near ER exit sites in almost all eukaryotic cells. The cisternal stack has 2 faces: a cis (entry face) and a trans face (exit face). Different enzymes are present in these 2 faces.
In the case of cis face, early modification enzymes are present while in the trans face of cisternae the enzymes required for the final processing of proteins are present. These enzymes are anchored to the membrane, whereas in ER they are anchored in the lumen.
Golgi Apparatus Function
The Golgi organelle is responsible for processing steps like glycosylation, packaging molecules into vesicles, production of lysosomes, and transport of lipids. In glycosylation, sugar monomers are attached to proteins in a series of steps.
Golgi Apparatus sorts the proteins emerging from ER and packs and tags them so that they can be allocated to their destinations.
The modified and tagged glycoprotein is packaged in form of vesicles for transport. They can be stored in form of vesicles for later use in the cell itself as in lysosomes.
Cells that have a secretory function have more Golgi apparatus and they are also larger in these mammalian cells. For instance in plasma B cells play a crucial role in secreting antibodies.
Biological Importance of Golgi Apparatus
The endoplasmic reticulum secretes proteins in form of vesicles for transport to Golgi where they are further modified. For instance, the oligosaccharides on lysosomal proteins are phosphorylated in cis cisternae of Golgi where the mannose residues are also removed. N-acetylglucosamine is added in the mid-cisternae stack.
In trans face, sialic acid and galactose are added. Other processes like sulfation of carbohydrates and tyrosines also occur here. Proteins are also tagged here to specify their destinations. For example, the proteins destined for the lysosome are labelled with mannose-6-phosphate.
The protein that is secreted by RER is enclosed in vesicles and transported to Golgi for maturation. The vesicles containing properly folded proteins pinch off from ER in this process.
They are then transported via cytoskeletons to the cis face for post-translational modifications. The vesicle fuses with Golgi and empties its contents into its lumen. Here the protein is processed, tagged with a signal sequence, and packed for export.
If the vesicle is to be exported to the extracellular space then it will fuse to the cell membrane for constitutive secretion. These vesicles fuse when they receive a signal, till then they are transiently stored. This process is termed as regulated secretion as in neurotransmitters released by neurons.
For hydrolytic enzymes like proteases, the vesicles are first shuttled to late endosomes and then transported to the lysosome.
Golgi Apparatus Citations
- Golgi Apparatus: An Emerging Platform for Innate Immunity. Trends Cell Biol . 2020 Jun;30(6):467-477.
- The Golgi apparatus and cell polarity: Roles of the cytoskeleton, the Golgi matrix, and Golgi membranes. Curr Opin Cell Biol . 2020 Feb;62:104-113.
- Form and function of the Golgi apparatus: scaffolds, cytoskeleton and signalling. FEBS Lett . 2019 Sep;593(17):2289-2305.
- Direct trafficking pathways from the Golgi apparatus to the plasma membrane. Semin Cell Dev Biol . 2020 Nov;107:112-125.
- Images are created with BioRender.com.
Share
Similar Post:
-
Sarcoplasmic Reticulum: Definition, Function, & Examples
Continue ReadingSarcoplasmic Reticulum Definition
Sarcoplasmic Reticulum is a smooth endoplasmic reticulum type found in striated and smooth muscles that are reserves of calcium ions.
What is Sarcoplasmic Reticulum?
The endoplasmic reticulum (ER) occurs as an interlinked network of flattened sacs in the cytoplasm. The membrane extensions of the organelle are connected to the outer nuclear envelope and also extend into the plasma membrane.
The ER is of two types based on their appearance: the rough endoplasmic reticulum (RER) and the smooth endoplasmic reticulum (SER). RER has ribosomes on their surface while SER lacks them. Sarcoplasmic reticulum refers to specialized SER found in muscle cells.
Sarcoplasmic Reticulum Characteristics
The sarcoplasmic reticulum is present in myocytes as a membrane-bound network of tubules surrounding the myofibrils abounds in the myocyte. Sarcoplasmic Reticulum is located proximal to the transverse tubules in cardiac and skeletal muscle cells. A triad is formed by the union of the sarcolemma that is an extension of the cell membrane and components of Sarcoplasmic Reticulum.
Terminal cisternae are formed by the close association of parts of Sarcoplasmic Reticulum with transverse tubules. It is the primary site responsible for calcium release in the muscle cell.
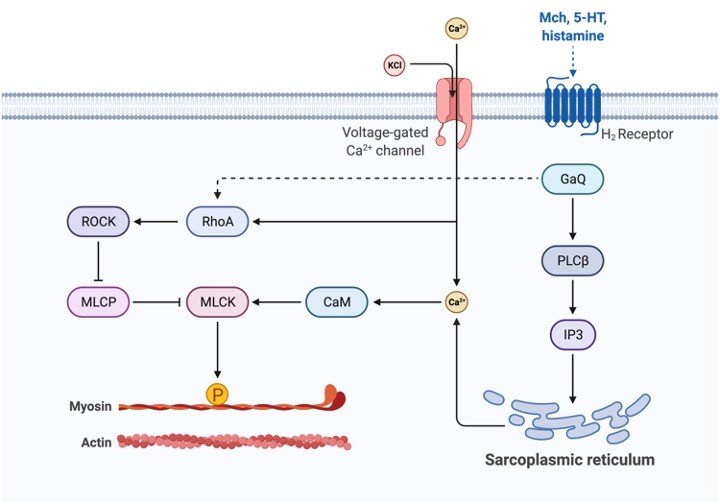
Sarcoplasmic Reticulum has ion channel pumps that help in an influx of calcium ions present on the membrane. These pumps are termed SERCA and consist of 13 subunits. For Sarcoplasmic Reticulum to carry out the role of absorbing calcium ions, it has ion channel pumps in its membrane.
These calcium pumps in the Sarcoplasmic Reticulum are called SERCA and consist of 13 sub-units (M1-M10, N, P, and A). Some subunits are located outside (Eg: N, P, and A) while the rest is present inside the membrane.
ATP binds to the extracellular subunit while Calcium ions bind to the intracellular subunits. SERCA2a is generally found in muscle cells.
Calsequestrin binds to calcium and helps in its storage. This protein is located in terminal cisternae and can bind to 50 Calcium ions at a time. Ryanodine receptors (RyRs) help in the release of calcium ions via Sarcoplasmic Reticulum. The most common receptor is RyR3 present in the brain, while RyR2 is located in cardiac muscles. RyR1 is seen in skeletal muscles.
Sarcoplasmic Reticulum Functions
The Sarcoplasmic Reticulum is crucial for the contraction of myofibrils and functions in the absorption, storage, and release of calcium ions. These ions are released when muscles contract while in the relaxation phase they are absorbed.
Calcium levels need to be regulated to maintain homeostasis. A maintained increase in calcium levels can cause hardening, calcification, and even cell death. The calcium levels are kept constant through Sarcoplasmic Reticulum. As it concentrates the calcium ions inside, it decreases its intracellular levels.
Importance of Sarcoplasmic Reticulum
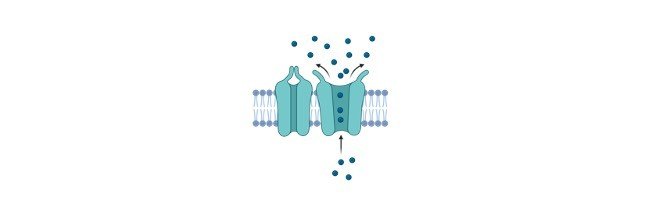
Calcium ion channel pumps ions into Sarcoplasmic Reticulum utilizing active transport as this is against the concentration gradient. Calcium is taken in when ATP along with 2 calcium ions binds to the cytosolic side of the pump.
The hydrolysis of ATP causes structural alterations of the pump. This leads to the opening of the channel through which the calcium ions pass through into the Sarcoplasmic Reticulum.
The calcium ions are released through the activation of RyR receptors at the terminal cisternae. This causes a surge in ion concentration causes calcium spark that can be spontaneous or evoked.
Calcium-induced calcium release is a mechanism for triggering calcium release. An action potential alters the shape of dihydropyridine receptors resulting in the opening of a calcium ion channel.
The resulting influx leads to the binding of calcium ions to RyR. This receptor is activated on binding with 4 calcium ions and this leads to calcium release. This results in the evoked calcium spark and is observed in the case of smooth and cardiac muscles.
In the case of skeletal muscle, RyR receptors open directly when dihydropyridine receptors change and open, and thus, this occurs without prior flooding of calcium ions. Both kinds of receptors are in proximity to each other, such that any change in dihydropyridine receptor shape triggers RyRs.
In cardiac and smooth muscles, the dihydropyridine receptors are not directly in contact with RyR, and hence calcium ion flooding and binding occurs is prerequisite for the release of calcium.
If no sort of action potentials are required for calcium release, then it is termed a spontaneous calcium spark. This mechanism is dependent on the high calcium ion concentration inside the SR.
The RyR opens by either direct binding of calcium ions or the detachment of calsequestrin from the receptor. If the concentration of calcium ions is low, then calsequestrin inhibits the opening of RyR by binding to it.
Sarcoplasmic Reticulum Citations
- Sarcoplasmic reticulum-mitochondria communication in cardiovascular pathophysiology. Nat Rev Cardiol . 2017 Jun;14(6):342-360.
- The sarcoplasmic reticulum and SERCA: a nexus for muscular adaptive thermogenesis. Appl Physiol Nutr Metab . 2020 Jan;45(1):1-10.
- Sarcoplasmic reticulum and calcium signaling in muscle cells: Homeostasis and disease. Int Rev Cell Mol Biol . 2020;350:197-264.
- Images created with BioRender.com
Share
Similar Post:
-

Trehalose: Definition, Function, & Examples
Continue ReadingTrehalose Definition
Trehalose consists of α-glucose molecules that form disaccharides and is a source of energy for organisms like fungi, plants, invertebrates, and bacteria.
What is Trehalose?
Carbohydrates are a sub-class of biomolecules that is categorized depending on the number of saccharide constituents. When 2 monosaccharides are linked by a glycosidic linkage they form a disaccharide i.e., a carbohydrate an example of a disaccharide is trehalose.
Trehalose History
It was H. A. L. Wiggers in 1832 who discovered trehalose in ergot of rye. But the name of this disaccharide was given in 1859 by Marcellin Berthelot based on its source trehala, which was extracted from the weevil’s pupal case.
Trehalose Properties
The monosaccharides in trehalose are linked by 1-1 α-glycosidic linkage. It has a white crystalline appearance and it is also known as α-D-glucopyranosyl-(1→1)-α-D-glucopyranoside and has a formula C12H22O11.
In terms of its components i.e., saccharides it is similar to maltose as it is comprised of 2 glucose units. But, maltose differs with respect to the glycosidic bond, it has α-1→4 glycosidic linkages.
Unlike trehalose where this bond occurs between C-1 of either glucose, on maltose the linkage occurs between C-4 and C-1. If the glycosidic linkage between 2 glucose units is β-(1→4), then they form cellobiose whereas if the linkage is α-(1→6) then isomaltose is formed. Unlike trehalose, maltose is a reducing sugar.
The closed ring structure in trehalose is due to the glycosidic bond between glucose units. Due to this, it is resistant to acid hydrolysis as the aldehyde or the ketone ends cannot bond with lysine or arginine of proteins.
This disaccharide is also stable at high temperatures and in acidic conditions. As it is an energy source and also has high retention trait organisms can endure long dry or freezing situations.
Trehalose Biosynthesis
The only anomer of trehalose is α,α-trehalose, and is naturally utilized by different organisms as an energy source. These organisms have inherent pathways and enzymes required to produce this disaccharide biosynthetically. One general metabolic pathway requires a trehalose-phosphate synthase enzyme.
From UDP-glucose the glucose unit is transferred to glucose-6-phosphate and the reaction is catalyzed by trehalose-phosphate synthase to produce threlaose-6-phosphate and UDP. Then, trehalose-P phosphatase catalyzes the dephosphorylation of trehaloses-phosphate.
Trehalose via trehalase enzyme is broken down into glucose units that provide energy in organisms like insects for flight. In the case of higher animals including humans, this reaction is catalyzed by α-glucosidase trehalase that is present in the brush border of cells of the intestinal mucosa.
Biological Importance of Trehalose
This natural disaccharide is biosynthesized in organisms like invertebrates, fungi, and plants. In these organisms, it acts as both the source of carbon and energy. It not only provides a fast energy source but also forms a structural entity in the cell walls of certain organisms and acts as an osmoprotectant.
Plants like Selaginella can survive long periods of dehydration, desiccation, oxidative stress, and heat by utilizing trehalose. During the dry spells it shrinks into a brown ball-like shape but on contact with moisture resumes growth. This phenomenon of resurrection is referred to as poikilohydric.
In omnivores and herbivores, sources like mushrooms, shellfish, and starchy food can deliver trehalose. This is broken down by trehalose that attacks the glycosidic linkage.
It can also be produced synthetically for commercial purposes to produce drugs, food, frozen foods, and cosmetics. In frozen foods like ice cream, this is added as an ingredient to decrease the freezing point of food.
It can also be used as an artificial sweetener as it does not cause a spike in glucose levels in the blood. It also promotes the growth of gut microbes like Clostridium difficile so consumption beyond the required amount is not advised.
Certain toxins are also released as a result of trehalose metabolism. Inborn error of metabolism Trehalase deficiency is a rare metabolic condition in the case of humans where the enzyme is not sufficiently produced and as a result, it accumulates.
It can lead to diarrhea and abdominal discomfort when ingesting dietary trehalose. This deficiency can result due to mutation in the TREH gene.
Trehalose Citations
Share
Similar Post:
-
Polyols: Definition, Function, & Examples
Continue ReadingPolyols
Polyols are primarily organic alcohol that has multiple hydroxyl groups. The name polyol is comprised of 2 terms, “poly” refers to multiple and –“ol” indicates alcohol group.
What are Polyols?
Polyols like other organic compounds comprise C-C and C-H covalent chemical bonds. Sugar alcohols are a group of polyols that are derived from sugars produced naturally or synthetically. Even though they are formed by the hydrogenation of sugars, they are not true sugars.
Sugars are comprised of monosaccharides and disaccharides having a formula Cn (H2O) n, where n may range from 3 – 7.
The group sugar alcohols are similar in structure to sugars except for the fact that they have additional hydroxyl groups and have the formula (CHOH)nH2. They are less sweet and can act as an energy source.
Polyols Pathway
Glucose is converted into fructose in the polyol pathway through a biological process. In this pathway, glucose is first reduced to polyol sorbitol that is oxidized to fructose. The first reaction is catalyzed by aldose reductase whereas the oxidation of sorbitol occurs in presence of the enzyme sorbitol dehydrogenase.
This pathway also employs cofactors, NADPH and NAD+. Dysfunctions in this pathway can lead to microvascular damage to kidney cells, retina, or nerves that are insulin-independent leading to type-II diabetes complications.
If glucose has not been phosphorylated by hexokinase then instead of the glycolytic pathway, it enters the polyol pathway where it is converted to fructose.
This occurs when there is a surplus of glucose due to which enzyme hexokinase becomes saturated and the excess glucose is diverted to the polyol pathway instead. Here it is reduced sorbitol and utilizes NADPH and NAD+.
These co-factors become less available for other significant pathways and metabolic reactions like glutathione and nitric oxide production. It also increases free radical oxygen species which can be damaged cells.
Biological Importance of Polyols
The most important function of polyols is to provide an energy source and it is involved in the polyol pathway where glucose is reduced. In this pathway, excess glucose is reduced to fructose which is required for processes like glycation and fructolysis.
Natural polyols occur naturally in fruits like apples, prunes, peaches, and in prunes, in forms like sugar alcohol sorbitol. Another naturally occurring polyol is found in bacterial cell walls as sugar alcohol ribitol.
Polyols may also act to maintain high intracellular osmolality as osmolytes in the kidney cells of higher organisms; some also act as antifreeze molecules.
Artificially polyols can be synthesized starch and sugar. They are used commercially as an alternative for sugar or as additives. They are employed in food products that are categorized as having no sugar content or low calorie. These sugar sorbitols except erythritol provide about 2.4 kilocalories/g.
They are not labeled as essential nutrients. But for diabetic patients, it can help them in regulating glucose levels in the blood. They can also act as a substitute for sucrose that is also a natural sugar. It is synthesized by plants like beets and sugarcane.
It is extracted from these sources and commercially processed as sugars utilized in various preparations. But unlike polyols, it is an essential carbohydrate that provides both fructose and glucose.
One of their disadvantages is that it has a high GI index of 65 when compared to sugar alcohols that have a GI of less than 10.2. GI of glucose is 100, while fructose has a GI of 25.
A high glycemic index is not preferred as it means that the substance can cause a substantial peak in blood glucose levels as in conditions of obesity and diabetes mellitus.
Sugar alcohols with a low GI are preferred and they do not promote tooth decay as they are not metabolized by mouth oral microbes. They are used commercially in the production of medicinal syrups, lozenges, and toothpaste as they do not produce any sort of acids that might trigger tooth decay.
One of their downsides is that they are poorly digestible which can lead to irritation and increased fermentation by gut microbe leading to bloating, flatulence, and diarrhea. These conditions can be triggered even in smaller amounts for sensitive individuals.
Polyols Examples
Sugar alcohols class includes maltitol, sorbitol, xylitol, isomalt, erythritol, glycerol, lactitol, hydrogenated starch hydrolysates, and mannitol.
The most common polyol is sorbitol that is found naturally in fruits like pears. It can also be produced artificially for commercial purposes for the production of sugar-free food products like sugar-free ice cream.
Maltitol is another commonly used alternative polyol that can replace sugars that are employed in manufacturing sugar-free products like baked goods. Another polyol that is commonly used in candies is isomalt that is a disaccharide.
Polyols Citations
- Review on the Impact of Polyols on the Properties of Bio-Based Polyesters. Polymers (Basel) . 2020 Dec 12;12(12):2969.
- A review of polyols – biotechnological production, food applications, regulation, labeling and health effects. Crit Rev Food Sci Nutr . 2020;60(12):2034-2051.
- A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv Nutr . 2017 Jul 14;8(4):587-596.
Share
Similar Post:
-
Cellular Respiration: Definition, Types, and Examples
Continue ReadingCellular Respiration Definition
For the production of ATP, molecules like glucose are oxidized, this is called as Respiration. It involves 3 stages and occurs at various positions within the cell. In aerobic as well as anaerobic condition, respiration can take place.
Cellular Respiration Steps
i. Glycolysis
In Glycolysis, a 6C hexose undergoes few steps and gets disintegrated into a 3C compound called the pyruvate. This process takes place in the cytoplasm and requires 2 ATP for this process and obtains 2 ATP out of the process. The hydrogen atoms from the glucose are taken by the cytochrome system.
Glucose molecule transforms into pyruvic acid is the last step in anaerobic respiration, however in aerobic condition it is further degraded into Krebs cycle compound. However, when not present it can be transformed to lactic acid.
ii. Krebs Cycle
From a single glucose molecule, more ATP can be created, in aerobic conditions. The pyruvic acid from the cytosol in glycolysis is moved to mitochondria, an organelle which has large area and is somewhat sausage shape and respiration will take place over here.
Pyruvic acid in aerobic condition it is further degraded into Kreb cycle 2C compound. Now the 2C compound will further attach to a 4C compound which is a part of the cycle.
In aerobic respiration, oxygen binds with carbon compound and CO2 is liberated. Thus, this is why animals liberate out CO2. Carbon compounds are oxidized by the enzymes and the hydrogen atom are given to the cytochrome system.
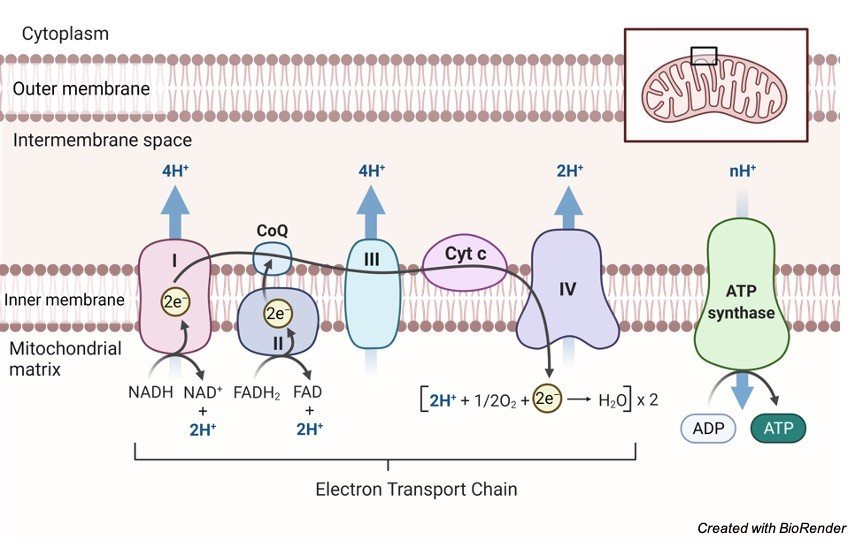
iii. Cytochrome System
The hydrogen carrier system, another name for cytochrome system, is the place where the hydrogen atoms are dispatched from kreb cycle and glycolysis. In the mitochondria is the cristae, where extremely small particles are present in the outer layer. The atoms are collected by the hydrogen acceptors.
The working of cytochrome system is as follows: where the previous coenzymes are consigned to the other coenzymes, which gets oxidized and liberates energy and hydrogen, these hydrogen will attach with the oxygen atoms of the aerobic respiration and will result in the formation of water molecule.
From one single molecule of glucose, 38ATP is gained in aerobic respiration. Glucose is also obtained from the food we consume, which can be utilized during respiration, however the mechanism differs. Through respiration plants also obtain energy.
Cellular Respiration Citations
Share
Similar Post:
-
Phosphorylation: Definition, Types, and Examples
Continue ReadingPhosphorylation Definition
Phosphorylation is the transfer of a phosphoryl group from a donor to a receiver molecule.
Phosphorylation is a biological process that involves the addition of a phosphate molecule to an organic substance such as glucose or adenosine diphosphate (ADP).
In the latter case, the addition of the phosphate group to transforms ADP to adenosine triphosphate (ATP), a crucial molecule that provides energy for a variety of functions in living cells, including nerve impulse transmission and muscular contraction.
The addition of a phosphate molecule to an organic substance is the easy response.
What is Phosphorylation?
Phosphorylation is a crucial biological step in which phosphate molecules are added to an organic component to make it useful for a variety of activities in a live organism.
Another similar term is “phosphorylate,” which refers to the addition of a phosphoryl group to an organic molecule.
The phosphorylation reaction is very important in biology since it is required for numerous biological processes including as apoptosis, inflammation, metabolic control, subcellular transport, and proliferation.
The transfer of phosphate molecules to a protein is known as phosphorylation in biology. In a living creature, this transfer prepares proteins for specific functions.
In humans, phosphorylation takes place on the side chains of three amino acids: (1) tyrosine (an amino acid produced in the body of living creatures from another amino acid called “phenylalanine”), (2) serine, and (3) threonine. However, histidine phosphorylation has also been shown in certain investigations.
Tyrosine, serine, and threonine are the amino acids that can be phosphorylated.
Phosphorylation is a reversible process, which means it may add and remove phosphate molecules. Kinases are the enzymes that are responsible for adding phosphate groups to proteins. The enzymes that remove these phosphate groups are known as “phosphatases.”
Purpose of Phosphorylation
Phosphorylation plays a crucial role during a sort of biological processes. It plays a role in the cell’s control of protein activities and signal transmission. Cell growth, signal transmission, cell development, protein synthesis, and cell division are all aided by it.
transmission, cell development, protein synthesis, and cell division are all aided by it. The reaction of phosphorylation is reversible. Phosphorylation and dephosphorylation (opposite of phosphorylation) activate and deactivate the majority of enzymes and receptors, respectively. Phosphorylation of enzymes acts as an on/off switch, changing the activity or function of the enzyme.
The phosphate molecule is a critical component of biomolecules. The researchers discovered that a huge number of metabolites (metabolite intermediates or end products) are phosphorylated in cells, resulting in the production of phosphates.
Initially, two scientists, Thomas Rall and Earl Sutherland, were doing study on the link between glucagon and epinephrine and therefore the liver enzyme glycogen phosphorylase.
The hormones in question were discovered to be involved in the synthesis of 3,5-cyclic adenosine monophosphate. This chemical was discovered to function inside a cell to alter the activity of phosphorylase. As a result, 3,5-cyclic adenosine monophosphate is a molecule that acts as a messenger for hormonal signals.
The phosphorylation of tyrosine regulates protein activity. Tyrosine is an amino acid that our bodies make from phenylalanine, another amino acid. The phosphorylation of the regulating tyrosine causes enzymatic activity-related modifications in the substrate. Phosphorylation can also be used for the following objectives;
1. Protein dissection
2. Control of Enzyme Inhibition
3. Glycolysis activity
4. Protein-protein communication
5. Controls energy-intensive chemical processes.
The Mechanism of Phosphorylation
Protein phosphorylation takes place on the side chains of three different amino acids, notably serine, threonine, and tyrosine, which are phosphorylated reversibly in eukaryotic cells.
The terminal phosphate group is targeted by the nucleophilic (–OH) group in these amino acids. The nucleophilic (–OH) group in these amino acids binds to the phosphate terminal group (-PO32-) and is transferred from the phosphate group to the amino acid side chain by the usual phosphoryl donor adenosine triphosphate (ATP), commonly known as ATP phosphorylation.
Magnesium (Mg2+) facilitates this transition, which lowers the phosphoryl transmission threshold in the γ- and ß-phosphate groups through the nucleophilic (–OH) group.
Due to the large amount of free energy created when ATP is disrupted to generate adenosine diphosphate (ADP) in the phosphate-phosphate bond, this phosphorylation process is one-way.
Phosphorylation is a critical step in controlling protein function across a wide range of proteins and is strongly connected to protein activity. By causing conformational changes in the phosphorylated protein, phosphorylation regulates protein function and cell signalling.
All of these phosphorylation changes have a two-fold effect on the protein. To begin, conformational changes in the protein are used to monitor its catalytic activity. As a result, phosphorylation can either activate or deactivate a protein.
Second, phosphorylated proteins attract surrounding proteins with structurally preserved domains that recognise and connect to phosphomotifs. The amino acids that differentiate these domains are distinct.
Phosphotyrosine (pY) selectivity is demonstrated by the Src homology2 (SH2) and phosphotyrosine-binding (PTB) domains, however variations between these two kinds are unique to various phosphotyrosine species.
Phosphoserine (pS) is recognised by both the MH2 and WW domains, but phosphothreonine (phosphorylated threonine) (pT) is recognised by forkhead-associated FHA domains.
The ability of phosphoproteins to attract other proteins, in which phosphorylated signal protein is recruited downstream, is important for signal transduction.
Protein phosphorylation is one of the reversible post-translational modifications (PTM) that is controlled by kinases to phosphorylate and phosphatases to dephosphorylate substrates. The phosphorylated protein dynamics behaviour in a cell is promoted by both enzyme types.
In reality, the size of the phosphoproteome in a single cell is determined by the spatial and temporal equilibrium of kinase and phosphatase concentrations inside the cell, as well as the catalytic performance at a specific location.
Protein Kinases
Kinases are enzymes that help phosphate transfer to substrates. More than 500 kinases have been predicted from the human proteome. This group of proteins can be found in the human kinome.
Lipids, carbohydrates, nucleotides, and proteins are among the substrates for kinase action. While guanosine triphosphate is utilised in a few kinases, ATP is nearly universally used as a co-substrate in protein kinases.
ATP is ideal for the transition of α-, β– or γ- phosphate classes in nucleotidyl-, pyrophosphoryl-, and phosphoryl conversion. The ATP binding site is generally preserved, although kinase substrate selectivity varies.
Protein kinases are divided into subgroups based on their catalytic domains, which include tyrosine kinases and serine/threonine kinases. Serine / threonine kinases account for about 80% of the mammalian kinome, while phosphoproteomes pS and pT account for more than 90% of the phosphoproteome.
According to research, the relative abundance ratio of pS:pT:pY in a cell is 1800:200:1. Despite the fact that pY is not as common as pS and pT, global tyrosine phosphorylation is at the top of biomedical science’s priority list due to its link to human sickness via the dysregulation of receptor tyrosine kinases (RTKs).
The protein kinase substrate’s specificity is determined not only by the desired amino acid, but also by the consensus sequences that surround it. In certain kinases, these consensus sequences allow for the phosphorylation of a single protein as well as many phosphorylated substrates (>300).
As a result, kinases may be able to phosphorylate single or multiple amino acids in individual proteins if kinase-specific consensus sequences are available.
Control subunits in kinases operate as activating or auto-inhibiting domains with various regulatory substrates. Phosphorylation of these subunits is a common method of regulating kinase activity. Most protein kinases are dephosphorylated and inactive in their natural state. Only a small percentage of phosphorylated kinases are fundamentally inefficient or inactive because they are constitutively active.
Phosphorylation and dephosphorylation must be combined in some kinases, including Src, indicating that this proto-oncogene is tightly controlled. By regulating the spatial connection between kinases and upstream and downstream regulators, scaffolding and adaptor proteins can also influence kinase behaviour.
The activity of certain kinases can be determined by incubating immunoprecipitate with substrates of different kinases and ATP. Commercial instruments are available for this type of test, and they can accomplish colorimetric, radiometric, or fluorometric identification.
Although this type of test shows the activity of specific kinases, including kinase enrichment, it does not show the proteins that kinases alter or the influence of endogenous phosphatase activity.
Signal Transduction Cascades
Because protein phosphorylation is reversible, cells may easily adjust to intracellular or external stimuli, this type of PTM is appropriate for signal transmission. One or more proteins that sensitively represent the physical sensors by binding a ligand, cleavage, or some other response differentiate signal transduction cascades of the signal.
When kinases are phosphorylated, these receptors activate downstream, then phosphorylate and activate their cognate downstream substrates, as well as other kinases, before reaching the exact response.
Signal transduction pathways can be linear, with kinase A activating kinase B, which then activates kinase C, and so on. Kinases A trigger multiple kinases, which in turn excite more kinases, revealing signalling pathways that amplify the initial signal. With this type of communication, a single molecule can induce widespread cellular activity like proliferation, for example.
Protein Phosphotases
The pace and breadth of phosphorylation-related signalling are controlled by three pathways:
1. Separation of the stimulating ligand from the other ligands
2. Proteolysis of a substrate by a kinase
3. Phosphatase Dephosphorylation
Around 150 phosphatases of pS, pT, and pY residue types are known to be present in the human proteome. Although both phosphatase groups have the same ultimate goal of dephosphorylation, they go about it in distinct ways.
Serine/threonine phosphatases, bimetallic centres (Fe/Zn), and tyrosine control quick hydrolysis in the phosphorus group of the phosphorus atom. Phosphatases produce a thiophosphoryl covalent intermediate that permits tyrosine residues to be removed.
In living cells, hosphorylation is crucial since it is through this process that energy-rich molecules (such as ATPs) are produced. Phosphorylases and kinases, for example, catalyse the addition of a phosphoryl group (phosphate) to ADP, resulting in ATP.
Phosphorylation Types
Phosphorylation and dephosphorylation may occur in a variety of substances.
1. Glucose Phosphorylation
When glucose is phosphorylated with different other sugars during its catabolism, it is referred to as phosphorylation. D-glucose is first transformed to D-glucose-6-phosphate in glycolysis, for example. Glucose is a tiny molecule that penetrates quickly. Because glucose is so little, it gets into the cells fast.
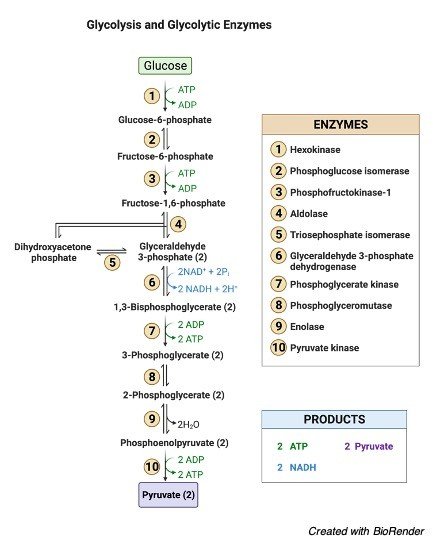
Phosphorylation is a larger enzyme that can’t get into the tissue rapidly. Phosphorylation is also necessary for the control of blood glucose levels. The synthesis of glycogen is directly proportional to the content of glucose. Phosphorylation of glucose is also linked to heart development.
2. Protein Phosphorylation
Phosphorylation of proteins occurs when an amino acid is linked to a phosphorylate group. Even though phosphorylation occurs with threonine and tyrosine in eukaryotes and histidine in prokaryotes, serine is the most common amino acid.
When one phosphate group interacts with the hydroxyl (-OH) group of a serine, threonine, or tyrosine side chain, this is referred to as an esterification process.
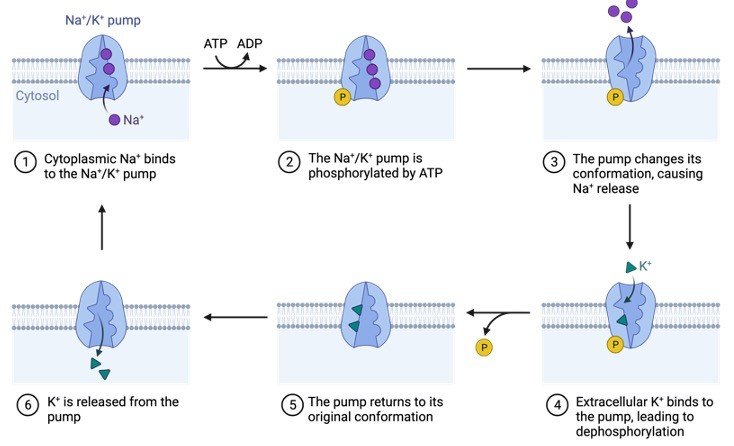
Protein kinase attaches the amino acid to the phosphate group in a covalent manner. The mechanism differs significantly between prokaryotes and eukaryotes.
The best-studied kind of phosphorylation appears to be post-translational modifications (PTM), which indicate that proteins are phosphorylated after conversion from an RNA sequence. Dephosphorylation and reversal processes were performed by protein phosphatases.
Protein phosphorylation may be seen in the phosphorylation of histones, which is a good example. In eukaryotes, DNA is linked with chromatin-forming histone proteins.
Histone phosphorylation alters the DNA-protein and protein-protein interactions, affecting chromatin shape. When DNA is degraded, phosphorylation occurs to provide new locations across damaged DNA so that repair procedures can operate correctly.
In addition to its importance in DNA repair, protein phosphorylation plays an important role in metabolism and signalling cascades.
3. Oxidative Phosphorylation
Oxidative phosphorylation is the process through which a cell releases and absorbs chemical energy. In the eukaryotic cell, reactions occur within the mitochondria. Interactions in the electron transport chain and chemiosmosis are involved in oxidative phosphorylation.
Finally, the transfer of electrons from molecules and proteins into the inner membrane of mitochondria via the electron transport chain produces the energy needed to generate adenosine triphosphate (ATP) in chemiosmosis.
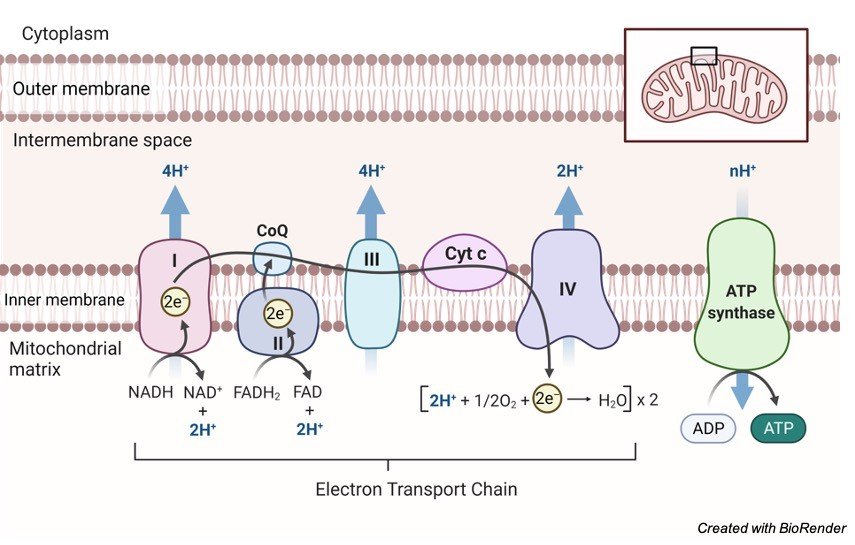
NADH and FADH2 send electrons to the electron transport chain during this procedure. As electrons go down the chain, they release energy as they go from higher to lower energy. Much of the energy used to create an electrochemical gradient is spent on transporting hydrogen ions (H+).
By interacting with H+ ions at the network’s end, electrons are transformed to oxygen and water is created. The ATP synthesiser receives ATP synthase energy from these ions.
As ATP is dephosphorylated, the phosphate group is degraded, producing energy that may be used by the cell. It’s possible that adenosine isn’t the sole base that gets phosphorylated to make AMP, ADP, and ATP. Guanosine, for example, has the potential to produce GMP, GDP, and GTP.
Detecting Phosphorylation
The phosphorylation of a molecule is detected using a mass spectrometer, electrophoresis, and antibodies. Phosphorylation sites, on the other hand, are difficult to identify and describe. Fluorescence, electrophoresis, and immunoassays are all employed in conjunction with isotope marking.
Biological Importance of Phosphorylation
Organic phosphates, which help living organisms develop tissues through anabolic chemical reactions, are important in the transfer of energy.
The importance of organic phosphates in metabolism was demonstrated and the findings were reported in Harden and Young’s study, which showed that when inorganic phosphorus was applied to the media and then converted to organic phosphate, the fermentation of glucose by cell-free yeast juice quickly improved.
Physiological Importance of Phosphorylation
Phosphoric acid is used to make the cell protoplasm. Phosphorylation is thus a crucial chemical activity for all cells. It’s also important for absorbing and metabolising a wide variety of foods.
The following is a list of its effects on several metabolic pathways:
In relation to Carbohydrates:
1. Phosphorylation aids carbohydrate absorption by the intestinal mucosa as well as glucose reabsorption by the kidneys. Hexose phosphate is produced in each of these epithelial cells. This molecule undergoes dephosphorylation, allowing hexoses to enter the circulation while leaving phosphoric acid alone.
2. Glycogen synthesis from glucose and glycogen breakdown into glucose are hypothesised to occur during phosphorylation in the liver and muscles.
3. Phosphorylation is common at all phases of chemical changes that occur after muscle contraction. Phosphorylation and dephosphorylation occur in this area in a cycle. Glycogen is broken down into lactic acid by a number of phosphorylated molecules.
In relation to Fats:
1. The absorbing epithelium produces neutral fats and phospholipids mostly during fat digestion. The enzyme phosphorylase is responsible for phosphorylation.
2. Phospholipids, particularly lecithin, are produced by the liver. The transfer of fat is an important stage. It also acts as the primary step of fatty acid oxidation. Fatty acid oxidation is a mitochondrial activity.
In relation to Proteins and Vitamins:
All phosphoproteins, including nucleoproteins, caseinogens, and others, must be produced. Phosphorylation is so expected to have a role in tissue oxidation, which is the process by which proteins, lipids, and carbohydrates are destroyed.
When it comes to the vitamin, it’s a no-brainer. Compounds like riboflavin phosphate, thiamine pyrophosphate, and others are found in certain vitamin B groups.
They are thought to be coenzymes in the oxidation and reduction phases of cell metabolism.
Regulation of Phosphorylation
The adrenal cortex is considered to be the source of the phosphorylation. Glucocorticoids increase the inhibition of phosphorylation after adrenalectomy.
Through its adrenocorticotropic hormone, the anterior pituitary may offer sophisticated phosphorylation control to the adrenal cortex.
The marasmic condition of adrenal cortex disorders may be explained in part by phosphorylation disturbance, which impairs absorption, metabolism, and body nutrition.
Phosphorylation Citations
Share
Similar Post:
-

Plants: Definition, Types, and Examples
Continue ReadingPlants Definition
As defined by botany, any eukaryotic species of the biological kingdom Plantae that is photosynthetic and has a rigid cell wall. Planta is a Latin word that means “sprout, shoot, cutting.”
Plants Characteristics
A plant is any eukaryotic organism that belongs to the Plantae biological kingdom. In the strictest sense, plants are embryophytes, that included vascular plants, liverworts, hornworts, and mosses. In certain less stringent references, green algae was considered a plant in certain less stringent references. Green algae include both unicellular and multicellular species with chloroplasts and cell walls.
Autotrophs are plants that eat just their own kind of food. Photosynthesis is how they produce their own nourishment. They can capture energy through the chloroplast’s green pigment (chlorophyll) and use carbon dioxide and water to create carbohydrates as food and oxygen as a by-product.
Plants are typically found at the top of the food chain since they are autotrophs. They’re referred to as “producers.” Other creatures, especially mammals, consume them as food. Animals, on the other hand, are heterotrophs, meaning they must devour other species to survive.
Some animals (especially herbivores) consume just plants, whereas others eat only meat or a combination of animal and plant matter. Plants do not rely on animals to develop and thrive since they are capable of producing their own nourishment.
A group of carnivorous plants (such as the Venus flytrap) that catch and devour animal prey, primarily when photosynthesis is difficult, is an exception.
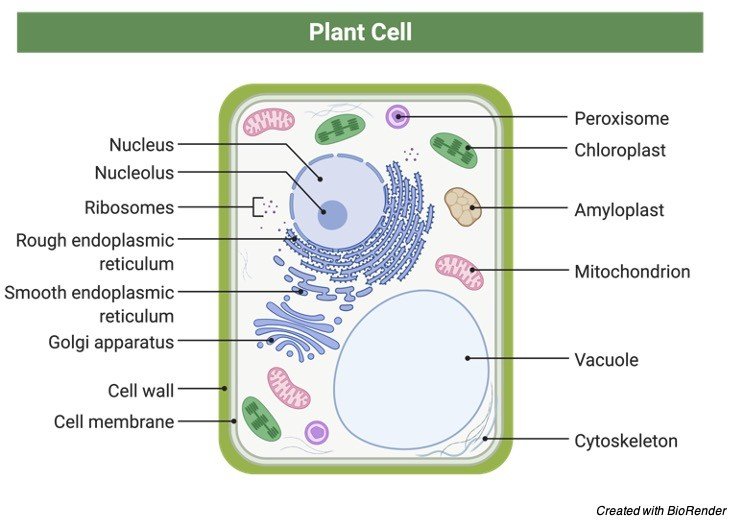
Plants are eukaryotes, or multicellular organisms. Plants, like mammals, have a membrane-bound nucleus inside their cells. The nucleus is an organelle that houses genes on chromosomes. Golgi apparatus, endoplasmic reticulum, lysosomes, peroxisomes, and plastids are further organelles suspended in the cytoplasm of a plant cell.
Plastids are found in plants. Plastids inside a eukaryotic cell indicate that it is more likely a plant than an animal. Plastids are available in a wide range of forms and sizes.
Chloroplasts are photosynthesis-related plastids that contain chlorophyll (green pigments). Apart from green, chromoplasts include pigments and are involved in pigment production and storage. Light energy is absorbed by chlorophyll systems at certain wavelengths in the electromagnetic spectrum.
Pigments also contribute to the colour of plant structures (e.g. green leaves, red flowers, yellow fruits). Non-pigmented plastids, such as amyloplasts, elaioplasts, and proteinoplasts, are known as leucoplasts. Their primary use is to store food. Sugar, such as starch, is used by plants to store food.
Inside the cell of plants, there is a huge vacuole. This cytoplasmic structure is important in turgor pressure control.
Apart from the plasma membrane, plants have stiff cell walls. A plant cell’s cell wall provides additional structural support.
Plants do not have a skeletal structure like animals, but their cell walls are largely made of cellulose material, which helps to provide structural support.
Plants have a unique cell division system in which daughter cells are separated by a cell plate (phragmoplast). Plants do not have the same mobility as mammals. They are unable to go from one area to another at their leisure. As a result, they must contend with extreme weather, such as heat. Their cell walls, which keep their bodies from drying up, are one of the ways they can endure heat.
Plants, meanwhile, continue to move, albeit in a different way. The folding of the leaflets of the plant Mimosa pudica when touched, for example, and the shutting of the leaf of the Venus flytrap while trapping prey are both examples of nastic movement.
Some plants, such as the silver birch Betula pendula, would even droop their branches and leaves at night, as if they were “sleeping.” Tropism is another type of plant movement.
Tropism, on the other hand, is more of a response to a stimulus than a movement. Plants, for example, prefer to grow towards the light source (phototropism).
Plasmodesmata are plasmodesmata found on plants. Plants contain plasmodesmata, which behave as if they were cell connections between plant cells, but animals have cell junctions that keep cells in an animal tissue.
These cytoplasmic bridges between neighbouring cells are formed by the cell wall. These “bridges” assist maintain the tonicity of plant cells by facilitating cell contact and allowing fluid circulation.
Plants are multicellular, with numerous cells organised into tissues and organs that work together to accomplish a specific purpose.
Anchorage, support, and photosynthesis are all specific functions of plant organs (e.g. roots, stems, leaves, etc.) Plants can grow indefinitely thanks to their meristematic tissues. Indeterminate, actively dividing cells make up the tissue, which gives birth to differentiated tissues including epidermis, trichomes, phellem, and vascular tissues.
Plants do not have sensory organs, yet they may detect their surroundings in a variety of ways. Despite their absence of eyes, ears, and noses, plants can “see,” “hear,” and “smell.” They appear to “feel” and respond in ways that animals do not.
Plants may not have the same nervous system as mammals, but they appear to have their own system based on how they respond to their environment.
Despite not having eyes, Arabidopsis has photoreceptors (at least 11 kinds) that let the plant perceive light. Herbivory, for instance, might cause the release of specific compounds on the damaged plant component.
Herbivores are deterred by the production of defensive compounds by plants. Tomatoes have been seen emitting volatile signals to warn adjacent plants of an imminent herbivore assault.
Plants reproduce in two ways: asexually and sexually. Plants reproduce asexually by budding, fragmentation, fission, spore development, vegetative propagation, apomixis, and other methods.
Male and female gametes merge during fertilisation, resulting in sexual reproduction. In general, the plant life cycle includes generational alternation, or the sporophyte and gametophyte stages alternating.
Plants are able to “breathe.” Carbon dioxide from the atmosphere enters the plant cell through stomata. Carbon dioxide is transformed to oxygen during photosynthesis, which the plant releases as a metabolic by-product into the environment through the stomata.
Plants may lack other biological systems, but they do generate compounds that aid in plant defence and immunological activities, as well as plant hormones that operate as signalling molecules.
Plant Body
Plant Body Embryophytes have two primary organ systems: one for the shoots and another for the roots. The shoot system comprises of body components in the plant’s top section, whereas the root system consists of body parts in the bottom region.
Plant parts such as stems, branches, leaves, flowers, and fruits may all be found in the shoot system. They are frequently seen above ground. Roots, tubers, and rhizomes are all part of the root system. They are frequently discovered underground.
Plant tissues include the following:
1. Plant tissues made up of undifferentiated and mitotically active cells are known as embryonic or meristematic tissues. Apical meristem and cambium are two examples.
2. Permanent tissues are differentiated cell-based plant tissues. Fundamental (e.g., parenchyma, collenchyma, sclerenchyma) and complex (e.g., parenchyma, collenchyma, sclerenchyma) permanent tissues can be further divided (e.g. phloem and xylem tissues)
3. Reproductive tissues are those parts of the plant that are engaged in reproduction. The sporogenous tissues are one example.
Plant cells are eukaryotic, meaning they have a well-defined nucleus. The chromosomes that carry genes are found in the nucleus. The endoplasmic reticulum, Golgi apparatus, mitochondria, lysosomes, and plastids are the other organelles except the nucleus.
Chloroplasts (containing chlorophyll, a green pigment), chromoplasts (with colours other than green), and leucoplasts (with pigments other than green) are the three types of plastids (colorless plastids). The vacuole is a huge structure inside the plant cell. It’s in charge of keeping turgor pressure in check.
The cytoplasm, where these organelles are suspended, is surrounded by the plasma membrane. The cell has a second layer, the cell wall, in addition to the plasma membrane. The cell wall, on the other hand, is not unique to embryophytes. Other species with cell walls include fungus, algae, and certain bacteria.
Primary and secondary cell walls make up the cell walls of embryophytes. Cellulose, hemicelluloses, and pectin make up a main cell wall. A thicker layer is the secondary cell wall. It contains a lot of lignin, which helps to reinforce and waterproof the wall.
One of the numerous functions of the cell wall is to aid in the resistance to osmotic pressure. Water flows into a plant cell when it is put in a hypertonic solution, causing the cell to expand. During severe osmosis, the existence of the cell wall prevents the cell from bursting.
Water diffuses out of a plant cell when it is put in a hypotonic solution, and turgor pressure is reduced, causing the cell to become flaccid. Further water loss will cause plasmolysis, which will eventually lead to cytorrhysis, or the total collapse of the cell wall.
Photosynthesis, respiration, transpiration, tropisms, nastic movements, photoperiodism, circadian rhythms, seed germination, and dormancy are all essential physiological activities that plants do.
Plant Genomics
Plants’ genomes are rather big. With roughly 94,000 genes, the wheat Triticum asestivum genome is the biggest of the plant genomes that have been sequenced.
Plant Life Cycle
Plants have two generations in their life cycle: gametophyte generation and sporophyte generation. Alternation of generations refers to the transition between diploid and haploid forms. Certain algae, such as Archaeplastida and Heterokontophyta, have this behaviour.
The sporophyte and the gametophyte are separate organisms in algae with generations that alternate. The gametophyte generation in embryophytes is one in which the phase begins with a haploid spore (n). To become a gametophyte, the spore goes through a sequence of mitotic divisions.
A haploid multicellular plant type is known as a gametophyte. There would just be one pair of chromosomes. Because the gametophyte phase is the sexual phase of the life cycle, the plant will grow sex organs that generate haploid gametes. Following the union of gametes, the gametes that participated in fertilisation would join the sporophyte generation, which is characterised by a diploid plant form.
The sporophyte is the main phase of tracheophytes (vascular plants) and is multicellular. As a result, the sporophyte is the most visible plant. The gametophyte, on the other hand, is the dominant plant in bryophytes (mosses and liverworts), and hence the predominant plant we see.
Tracheophytes’ life phases begin with a seed, which develops into a scion when conditions are favourable for development. The scion develops leaves, stalks, and branches as it grows. It grows into an adult plant that produces blooms in the end.
Sex cells are found in the flowers, such as sperm cells in pollen grains and ova in the ovary’s ovules. The seed contains a zygote formed by the fusion of the sex cells. Plants that are monoecious have both sex cells, whereas dioecious plants only have one kind of sex cell.
Plants can reproduce asexually as well. They achieve this by avoiding the use of gametes. Budding, fragmentation, fission, spore generation, vegetative propagation, and apomixis are all examples of asexual reproduction.
The ageing process of plants is referred to as plant senescence. During leaf senescence, for example, the yellowing of leaves happens as a result of chlorophyll breakdown, leaving only the carotenoids. However, certain plants, such as deciduous trees, may continue to produce new leaves.
Plant Ecology
Plants do not need to hunt or eat animals for nourishment since they are capable of photosynthesis (with the exception of carnivorous plants). They can create their own food by combining light energy, atmospheric carbon dioxide, and water molecules.
Nonetheless, the waste that animals exhale during breathing is one source of carbon dioxide. They give out oxygen as a waste product of photosynthesis in exchange. Animals, like other aerobic creatures, require oxygen to survive.
Other essential nutrients are obtained by plants from dissolved minerals in the soil. They take them in via their roots.
Calcium, magnesium, nitrogen, phosphorus, potassium, and sulphur are some of the macronutrients they get from the soil. Plants absorb boron, chloride, copper, iron, manganese, and molybdenum as micronutrients.
As a result, the breakdown of dead plant parts, or the entire plant, results in the return of important minerals and chemicals to the Earth.
They are frequently put at the top of a food chain due to their feeling of independence. In an ecosystem, they are the primary producers. As a result, plant extinction can have a significant influence on an ecosystem.
The Red List of Threatened Species of the International Union for Conservation of Nature (IUCN), a system for monitoring the conservation status of species across the globe, used a method of categorising species based on extinction risk. As a result, species can be classified as “data deficient,” “near-threatened,” “vulnerable,” “endangered,” “critically endangered,” “regionally extinct,” “extinct in the wild,” and “extinct.”
In 2016, the International Union for Conservation of Nature (IUCN) listed 2,493 plants as critically endangered and 3,654 species as endangered.
Plants develop symbiotic relationships with other species. Here are several examples:
1. Mutualism – for example, plants provide nectar for honeybees, while honeybees aid in the dispersion of pollen grains.
2. Predation – for example, carnivorous plants that eat insects and other tiny animals
3. Competition – for example, plants that compete for available space and nutrients with other plants for habitat.
4. Commensalism – for example, plant fruits that adhere to animal hair for free transportation.
5. Parasitism – for example, parasitic plants that obtain nutrients from their hosts, such as Cuscuta (dodder), which attaches to an acacia tree and develops haustoria that absorb nutrients.
The Census of Marine Life projected in 2011 that there might be about 8.7 million eukaryote species on Earth, including about 298,000 plant species. There have previously been 215, 644 described and catalogued.
Plant Evolution
Organelles such as plastids and mitochondria, according to the endosymbiotic hypothesis, reflect once free-living prokaryotes. The chloroplasts appear to be connected to cyanobacteria, which are prokaryotic microorganisms.
The structural similarity between cyanobacteria and chloroplasts provides the foundation. Furthermore, they share the same photosynthetic pigments as the genome and a single circular DNA molecule.
Endosymbiotic processes, it appears, were responsible for the emergence of the earliest photosynthetic eukaryotes one billion years ago. The embryophytes are thought to have evolved from Charophyta (a kind of green algae).
Many similarities exist between charophytes and embryophytes, such as the development of phragmoplasts during mitosis.
The following is a short chronology of embryophyte evolution:
Phanerozoic aeon » Paleozoic era » Ordovician period: The first embyophytes (land plants) arose during the Ordovician epoch (485 million years ago to 440 million years ago).
Phanerozoic eon » Paleozoic era » Devonian period: Primitive plants, trees, and shrub-like forests dominated the earth throughout the Devonian epoch (415 million to 360 million years ago), providing new habitats for terrestrial creatures. Elkinsia, an early seed fern, developed seeds, especially in the late Devonian era.
Phanerozoic eon » Mesozoic era: This period lasted 252 million to 66 million years. Flowering plants first occur in the Triassic period (about 200 million years ago).
Phanerozoic eon » Cenozoic era: The “new life” epoch, which stretches from 66 million years ago to the present day, is the most recent geological epoch. The grasses first developed during this time period, about 40 million years ago. To withstand the low CO2 and dry conditions of the tropics, these plants and many other plant species developed a novel metabolic process.
Plant Taxonomy
Because they all include chloroplasts and a cell wall, green algae, fungus, and embryophytes are included in the original definition of plants. Algae and fungus, on the other hand, were finally assigned to their separate kingdoms.
Plants (i.e. Plantae sensu strictissimo) are multicellular organisms having cellulose-containing cell walls and chloroplasts for photosynthesis in the strictest meaning. The kingdom Plantae is made up of embryophytes, which include vascular plants, liverworts, mosses, and other fossil plants with similar characteristics.
Embryophytes and green algae are included in Plantae sensu stricto (“plants in a limited sense”) (Chlorophyta and Charophyta). This concept of plants is still commonly used today. They belong to the Viridiplantae (or Chlorobionta) group, which is also known as the green plants.
The following are the divisions of the kingdom Plantae sensu stricto: Chlorophyta, Charophyta, Marchantiophyta (liverworts), Anthocerotophyta (hornworts), Bryophyta (mosses), Lycopodiophyta (club mosses), Pteridophyta (ferns, whisk ferns, and horsetails), Cycadophyta (cycads), Ginkgophyta (ginkgo), Pinophyta (flowering plants).
Significance of Plants
Plants are necessary for the survival of many creatures since they are the food chain’s producers. They are capable of storing starch. They’re also a valuable source of minerals and chemicals.
Plants provide homes for a variety of creatures, e.g. insects and arboreal organisms. They are also the most important source of oxygen for aerobic creatures.
Medicinal qualities can be found in some plants. Plantain (Plantago major) leaves for decreasing inflammation and discomfort, and burdock (Arctium minus) roots and leaves for eczema and damaged skin are just a few of the many therapeutic plants.
Essential oils, pigments, resins, tannins, alkaloids, amber, waxes, cosmetics, plastics, rubber, varnish, lubricants, inks, and a variety of other goods are all made from plants.
Buildings, musical instruments, boats, and furniture are all made from plant-based wood. It’s also utilised in the production of paper.
Plant Research
Botany is the field of science that examines plants (or plant biology). A botanist is a specialist in this subject. Some of the disciplines of study comprise morphology, cytology, histology, physiology, ecology, evolution, taxonomy, and pathology. Sub-disciplines emerged as a result of the diversity of plant groupings, including:
1. Paleobotany is the science of studying fossil plants.
2. Algology is the study of algae.
3. Mycology is the study of fungi.
4. Bryology is the study of mosses, liverworts, and hornworts.
5. Pteridology is the science of ferns.
6. Pollen grains and spores are studied in palynology.
Applied botany is concerned with the commercial and economic applications of plants. Agriculture (for example, agronomy, horticulture, and plant breeding), forestry (for example, dendrology, wood technology), pharmaceutical botany, and landscape architecture are all included.
Plants Citations
- Allelopathic Plants: Models for Studying Plant-Interkingdom Interactions. Trends Plant Sci . 2020 Feb;25(2):176-185.
- Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev Cell . 2020 Dec 7;55(5):529-543.
- Unraveling salt stress signaling in plants. J Integr Plant Biol . 2018 Sep;60(9):796-804.
Share
Similar Post: