Category: Biology
-
Cytidine Monophosphate: Definition, Types, and Examples
Continue ReadingWhat is Cytidine Monophosphate?
Cytidine monophosphate is nucleotide and can be written as C9H14N3O8P and these nucleotides comprises of 3 parts; phosphate group, sugar of 5C and a nucleobase.
Sugar can be either ribose or deoxyribose. DNA is made from the deoxyribose sugar and RNA from ribose sugar. The components of nucleic acids are the nucleotides.
The nucleic acid backbone is formed from phosphate and the sugar molecule and the synthesis takes place in 5’-3’ direction, although the two strands run in the opposite direction, so that they can bond with each other due to complementary bases.
Nucleotides can be linear as well as in cyclic form, where the phosphate group is bounded to the hydroxyl group of sugar. Phosphate group and nucleotide forms nucleoside.
Thus, to the sugar molecule, when a single phosphate group is attached it is called as nucleoside monophosphate and when two phosphate groups, it is called as nucleoside diphosphate and similarly when 3, it is called as nucleoside triphosphate.
Nucleoside can be deoxyribonucleoside or ribonucleoside. On the basis of pentose sugar, thus ribonucleoside consists of ribose sugar with nucleoside and these nucleosides can be adenine, guanine, cytosine and thymine.
Similarly, deoxyribonucleoside may have nucleosides like adenine, guanine, cytosine and thymine, in which adenine pairs with thymine and cytosine with guanine.
Pyrimidine and purine are two types of nucleoside where pyrimidine is single stranded and purine is double stranded.
Cytidine Monophosphate Structure

Cytidine monophosphate is an organic compound which contains ribose sugar, phosphate group, and nucleosides. As cytidine is bound to the ribose sugar, it is a pyrimidine base, with one phosphate molecule attached to the nucleobases.
Cytidine Monophosphate Synthesis
Through de-novo synthesis, CMP can be generated. Pyrimidine like cytosine can be formed through various steps. Carbamoyl phosphate synthetase forms carbamoyl phosphate which gets transformed to carbamoyl aspartate and then to dihydroorotate which will further be oxidized to orotate, which interacts with PRPP to form orotidine-5- monophosphate, which is transformed into pyrimidine.
For the synthesis of uridine mono phosphate, from OMP carbon dioxide is removed by OMP decarboxylase. Uridine mono phosphate is phosphorylated to form Uridine di phosphate and similarly triphosphate, which when aminated forms cytidine triphosphate by CTP synthetase.
Cytidine monophosphate is formed when CTP is disintegrated. Breakdown of further CMP results in formation of cytosine and end-products like ammonia, CO2 and Beta-alanine.
Through salvage pathway cytosine can be re-obtained. Through deamination, cytosine is transformed to uracil and then to uridine with uridine phosphorylase and further with the help of nucleoside kinase, uridine is transformed to UMP.
Cytidine Monophosphate Function
Monomer of RNA is cytidine monophosphate, where CMP is reduced to deoxycytidine monophosphate and phosphorylated to form cytidine diphosphate by CMP kinase and when again phosphorylated it produces cytidine triphosphate.
Cytidine Monophosphate Citations
- Bacterial CMP-sialic acid synthetases: production, properties, and applications. Appl Microbiol Biotechnol . 2008 Oct;80(5):757-65.
- Cytidine 3′,5′-cyclic monophosphate: a third cyclic nucleotide intracellular mediator? Biochem Soc Trans . 1992 May;20(2):469-74.
- Cyclic cytidine 3′,5′-monophosphate (cCMP) in cell regulation. Mol Cell Endocrinol . Nov-Dec 1982;28(3):373-85.
Share
Similar Post:
-
Facilitated Diffusion: Definition, Types, and Examples
Continue ReadingFacilitated Diffusion Definition
Facilitated diffusion (also known as facilitated transport or passive-mediated transport) is the process of spontaneous passive transport (as opposed to active transport) of molecules or ions across a biological membrane via specific transmembrane integral proteins.
What is Facilitated Diffusion?
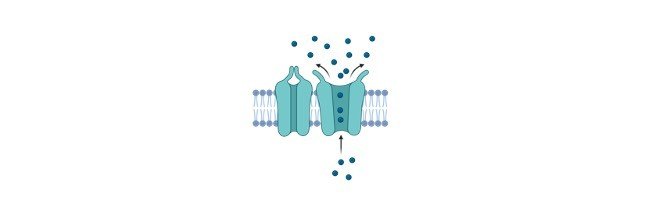
Facilitated Diffusion is a passive transport where the movement of molecules is mediated through the plasma membrane with the help of transporter protein such as carrier is called as facilitated diffusion.
The transfer of molecules takes place from a higher concentration area to a lower concentration in facilitated diffusion as well, like simple diffusion. The solute concentration variation through the membrane is the driving force responsible for facilitated diffusion.
Although majority of facilitated diffusion does not suffice the need of ATP, however in few cases it does require ATP. Facilitated diffusion take place due to the exactitude between the carriers and the solute.
In facilitated diffusion molecules can progress in both the direction i.e., towards or against the concentration gradient. Kinetic energy along with concentration gradient helps to carry out facilitated diffusion.
The molecules which can pass through are water soluble huge molecules through the plasma membrane in facilitated diffusion.
Facilitated Diffusion Principle
Plasma membrane’s lipid bilayer is hydrophobic, thus water-soluble molecules cannot pass through; however tiny water molecules due to their concentration gradient can pass through the membrane. Although hydrophobic huge molecule requires either carriers or channel protein to pass through the membrane.

When molecules passes with the help of the channel protein, there are pores present in the transmembrane of the membrane, resulting in the flow of molecules and are spread throughout the cytosol and outer surrounding thus, extending to the other organelles.
Through the transmembrane channel, charged molecules will move and these transporters protein are incorporated to the membrane, having attraction towards the matrix.
However, in other cases, molecule will attach to the carrier protein, which will change the shape of the molecule leading to the molecule move inside the cytosol, and this process is seen in enzymes, which are huge molecules.
Facilitated Diffusion and Channel Proteins
These proteins aid in the movement of molecules, by channel formation through the membrane and in this membrane lies the transmembrane proteins which are ions.
These channels have a diameter of 4-5 Armstrong and can be choosy and allow only a particular ion to move through such as positive ion and will have differences for various ions, thus being picky towards a particular ion. In the extra and intracellular matrix, hydrophilic domains and core are possessed by these channel, thus cracking the layers wide open.
The water movement through the membrane is mediated by the aquaporins at a very faster pace. On the receival of the electrical signal or attachment of molecule, the doors gets closed and open.
Facilitated Diffusion and Carrier Proteins
Carrier proteins perform facilitated diffusion, by transporting the molecules through the membrane. They do so by attaching to the molecule and getting changes in its shape as they are heavy and then it can move within the cell on the basis of the concentration.
The change in shape also has an impact on the hydrogen bonds. Carrier protein are quite specific in terms of their binding sites, such as they recognize between the D and L sugar type, which makes the plasma membrane as well specific.
Saturation occurs when carrier protein attaches to the substrate, thus allowing movement to take place at a faster pace. They play role in active transport which requires energy for the transportation of molecule.
Factors Affecting Facilitated Diffusion
Environmental factors are the one which has an impact on facilitated diffusion:
a) Concentration Gradient: The diffusion through the membrane occurs due to concentration gradient, which happens from a high concentrated region to a lower concentrated one. However quick diffusion happens due to the concentration diffusion.
b) Temperature: For the shape to change, the amount of energy required is quite high than the activation energy. As the temperature increases the carrier transportation also increases, thus elevating the reaction rate between the carrier protein and the substrate in the molecule.
c) Saturation: On the membrane, carrier proteins are present in specific amount and once these sites are occupied, no more proteins can bind. Thus, even if concentration gradient rate is elevated, rate of diffusion cannot be elevated.
d) Selectivity: Selectivity and transportation rate are reciprocal to each other, as selectivity is achieved from the binding sites which do not treat all the solutes equally, thus ceasing the movement.
Facilitated Diffusion Examples
1) Glucose and amino acid transport: Example is the movement of glucose and amino acid from blood to the cell is the facilitated diffusion example. Through active transport they reach the intestine and are left into the blood. These molecules are moved to cell from the blood through carriers such as amino acid permease and glucose transporters, as they are quite huge.
2) Gas transport: A different example is when to the muscle and the blood, oxygen is transported. The carrier protein is hemoglobin blood and myoglobin in muscle, which results in diffusion due to increase in pressure and thus gets moved to the other side with low pressure. The same process is involved in carbon monoxide and dioxide.
3) Ion transport: Ions possess the same charge like the membrane and are polar thus, cannot move through and thus have transmembrane protein also known as ion channel which are choosy for ions like Na, K and Ca and the transport has to be quick as no energy is used.
Biological Importance of Facilitated Diffusion
The homeostasis between the outer and inner environment is regulated by facilitated diffusion. It also makes the biological membranes specific. Various functions are regulated by facilitated diffusion such as ion transport, oxygen transport and transportation of sugar molecules.
Facilitated Diffusion Citations
- Facilitated diffusion in chromatin lattices: mechanistic diversity and regulatory potential. Mol Microbiol . 2005 Aug;57(4):889-99.
- Facilitated Diffusion Mechanisms in DNA Base Excision Repair and Transcriptional Activation. Chem Rev . 2018 Dec 12;118(23):11298-11323.
- Facilitated diffusion of Argonaute-mediated target search. RNA Biol . 2019 Sep;16(9):1093-1107.
Share
Similar Post:
-

Frameshift Mutations: Definition, Mechanism, and Examples
Continue ReadingFrameshift Mutation Definition
Frameshift mutations occur when nucleotides in the coding region are inserted or deleted, resulting in an altered amino acid sequence during codon translation. A phenotypic alteration, such as the synthesis of an altered protein, may occur from this sort of mutation.
What is Frameshift Mutation?
Frameshift mutation is a form of gene mutation in which the addition or deletion of one or more nucleotides produces a shift in the reading frame of the codons in the mRNA, which can result in an amino acid sequence change during protein translation.
Frame shift mutation is a variant. Reading frame mutation, reading frame shift or framing mistakes are all synonyms for frameshift mutation.
Causes of Frameshift Mutation
The nucleotides of a nucleic acid (such as DNA) can be “read” in groups of non-overlapping, consecutive triplets known as a reading frame. Triplets (or codons) in a reading frame are translated into particular amino acids during translation (or a codon signal).
As a result, if a mutation occurs, such as an insertion or deletion of a nucleotide, the reading frame may be altered. The amino acid sequence is entirely altered. Such changes are known as frameshift mutations (also called reading frame mutation, reading frame shift, or framing error).
The deletion of two nucleotides, which in the above diagram are the nucleotides containing bases, cytosine (C) and guanine (G), has resulted in an erroneous amino acid (G). Glutamate has taken the role of arginine (arg) (glu).
The reading frame is unaffected by the insertion or deletion of nucleotides in multiples of three. As a result, the protein in these situations is likely to have an additional or missing amino acid.
Frameshift mutations are most commonly generated by a mutational mistake during DNA replication or repair. Exposure to acridine dyes, which are capable of causing frameshift mutations, can also cause them. The reading frame of the nucleotide sequence changes due to insertion or deletion (also known as indels) of the nucleotide.
However, the consequences of these mutations vary depending on where they occur. At the interstitial or intercalary location, a nucleotide can be added or deleted.
In certain cases, the insertion and deletion of nucleotides happen at the same time (known as double frameshift), restoring the reading frame to its original state.
A frameshift mutation can cause the whole structure and function of a protein to be lost, resulting in a non-functional polypeptide. However, the phenotypic impact of the mutation will be decided by the resultant codons, post-mutation, and mutation location.
There are three sorts of codons that occur from frameshift mutations;
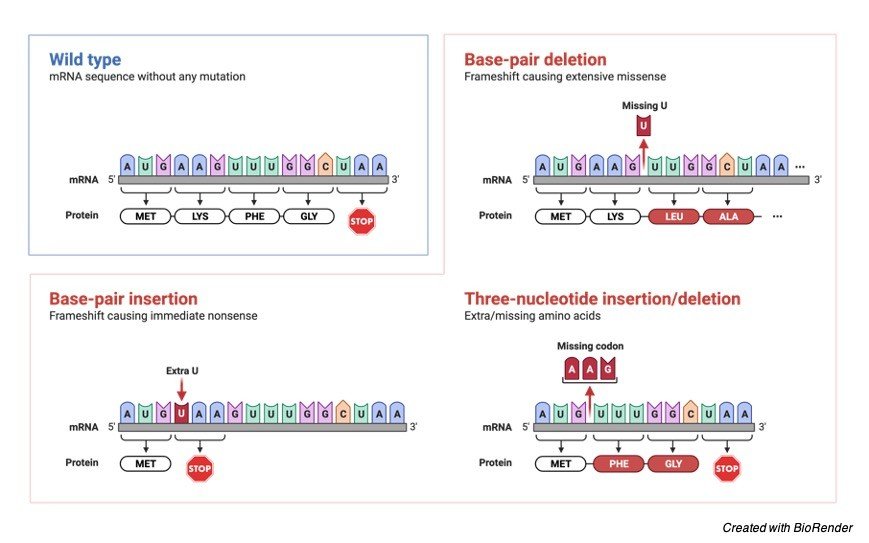
1. Sense Codons: are codons that are read in the same way they were before frameshift mutation.
2. Missense Codons: these are codons that result in the production of an erroneous or different amino acid.
3. Non-sense Codons: these are codons for which no matching tRNA exists, causing the translation process to be truncated.
As a result, frameshift mutations produce an aberrant or faulty protein product with an incorrect amino acid sequence. Such proteins may be entirely new or unusable, depending on where the mutation occurs. A stop codon can also occur from a frameshift mutation. The premature stop codon on mRNA will interrupt the translation process, resulting in a short polypeptide.
The protein may be shorter or longer than the normal protein, depending on the degree and type of the frameshift mutation. Such mutations might arise naturally or as a result of external stressors.
Frameshift mutations are more common in Adenine-Thymine (AT)-rich areas of the nucleic acid, which is an intriguing finding.
Types of Frameshift Mutations
Frameshift mutations can arise when a nucleotide in the nucleic acid is deleted or inserted. A Deletion frameshift mutation occurs when one or more nucleotides in a nucleic acid are deleted, causing a shift in the nucleic acid’s reading frame, or reading frameshift.
Deletion is a more typical method for causing a frameshift mutation, which causes an altered reading frame. (+) 1 frameshift mutation is another name for this mutation.
Insertion frameshift mutation occurs when one or more nucleotides are added to the nucleic acid’s base sequence, causing a shift in the reading frame. The number of nucleotides and the position of nucleotide insertion determine the severity of this sort of frameshift mutation.
The (-) 1 frameshift mutation is another name for this mutation. Understanding frameshift mutations that arise when a nucleotide is inserted or deleted from the usual nucleotide sequence.
Effects of Frameshift Mutations
Frameshift mutations can lead to the following outcomes:
1. A protein with a changed coding sequence may be unusable or a totally different protein. As a result, a variety of metabolic processes may be disrupted.
2. An abrupt halt to the translation process results in non-usable protein, which has ramifications for the physiological systems involved.
3. A frameshift mutation can also lead to cellular translational process abnormalities. The cellular machinery may correct the mistake by upregulating the expression of the mutant gene if no functional protein is produced owing to frameshift mutation. This can cause the cell’s translation machinery to malfunction. As a result, a high number of misfolded proteins may develop, which might be fatal to a cell.
4. However, as seen in HIV patients with frameshift mutations in the chemokine receptor gene, the altered protein might be advantageous and give protection (CCR5).
5. Frameshift mutations cause Crohn’s illness, cystic fibrosis, and some kinds of cancer.
The Genetic Code
The nucleotides encode all of the genetic information in RNA and DNA. A three-nucleotide sequence contains genetic information. Each nucleotide triplet is eventually translated into particular proteins that are necessary for diverse biological activities. There are two crucial stages in the translation of genetic information into protein.
1. Transcription: The genetic information encoded on DNA is “rewritten” on RNA in this process.
2. Translation: In this case, the transcribed RNA is “translated” into a particular amino acid sequence, which finally forms a polypeptide or protein chain.
Discovery of the Genetic Code
The transmission of genetic characteristics in Gregor Mendel’s early genetic studies suggested that genetic information is passed down from generation to generation as a discrete physical and chemical entity. Amino acids were later considered to be genetic information carriers.
The codons or triplets on the DNA sequence were found by scientists including Francis Crick, Sydney Brenner, Leslie Barnett, and Richard Watts-Tobin. Marshall Nirenberg, Heinrich J. Matthaei, and Har Gobind Khorana (1961-1964) discovered and deciphered the nature of a codon.
Reading Frames and Triplet Codon
The whole genome is split into three three-nucleotide segments that do not overlap. The reading frame is defined by the triplet codon that starts the translation process. A particular amino acid or a stop signal known as a codon is encoded by each triplet of the nucleotide. Twenty amino acids are encoded by 64 codon combinations.
However, three of these 64 codons are stop codons, resulting in 61 codons coding for amino acids and three codons coding for the end of the translation process (i.e., 61 codons for amino acids + 3 stop codons = 64 codons).
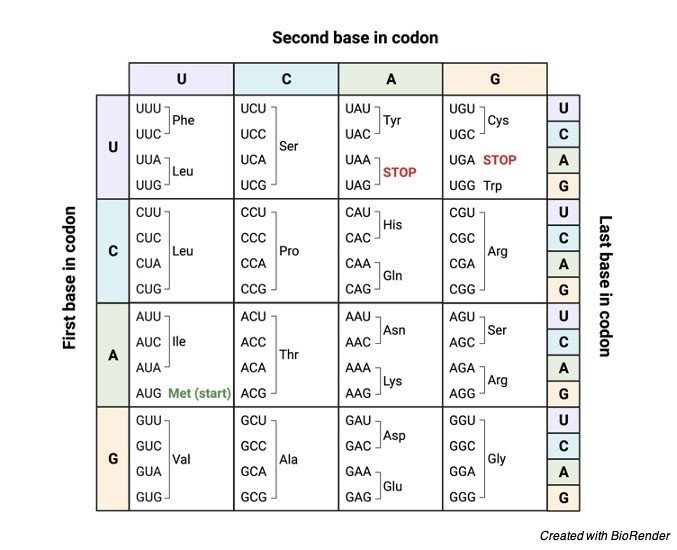
The following are some of the characteristics of a codon:
1. Each codon codes for a specific amino acid during translation. The beginning of amino acid synthesis, as well as methionine synthesis, is encoded by the AUG codon.
2. The three “stop” codons are UAG, UGA, and UAA, which signal the end of amino acid synthesis.
3. Codons are a universal language.
4. The translation process begins with a start codon and continues until the stop codon occurs on mRNA.
From the N-terminus (methionine) to the C-terminus, mRNA is encoded from 5′ to 3′, and it translates into an amino acid in a protein.
Ribosome Translocation
Each codon is converted into an amino acid by mRNA. The ribosomes then link these amino acids together in a process known as ribosome translocation. Protein synthesis is a cyclic process in which the ribosome advances three bases after adding one amino acid to the expanding chain of the polypeptide (i.e., one codon). The function of proteins and polypeptides is disproportionately affected by ribosome mobility.
Frameshift Mutation Examples
Let’s look at a base sequence in RNA that codes as follows to better understand frameshift mutations:
AUG-AAT-AAC-GCU = start-leucine-asparagine-alanine
If an A nucleotide is added or inserted after the start codon AUG as a result of a mutation in the aforementioned sequence, This will alter the reading frame fully:
AUG-AAA-TAA-CGC = start-lysine-isoleusine-alanine
As can be seen, adding only one nucleotide to the RNA sequence entirely changed the base sequence, resulting in the production of completely new amino acids during translation.
Other Examples
The coding sequence for a particular polypeptide is read continuously from the start codon AUG to one of the three stop codons in the reading frame of any mRNA.
The ribosome interprets whichever codons follow the start codon as it travels along the mRNA three bases at a time during translation. The usual reading frame can be disrupted by adding or removing one or two bases (or any other number that is not a multiple of three), resulting in the creation of a fully non-functional protein.
A premature stop codon can also be introduced by frame changes.
Original coding sequence: atggtgcatctgactcctgaggagaagtct
Amino acid translation is M V H L T P E K S
Frameshift: atggtgcctgactccTGAggagaagtct
Amino acid translation is M V P D S * G E V X
Frameshift Mutation Diseases
Mutations are a source of diversity; yet, some mutations are harmful and result in illness. Frameshift mutations have been linked to the following diseases:
1. Tay-Sachs Disease: Tay-Sachs disease is caused by a frameshift mutation in the Hex-A gene. In the absence of Hex-A, aberrant lipid build-up in the brain occurs. The lipids build up in the neurons and finally kill them. This is a deadly illness.
2. Cystic Fibrosis: Cystic fibrosis is caused by two frameshift mutations in the CFTR genes (one involves the insertion of two nucleotides and the other involves the deletion of one nucleotide). The CFTR gene controls the correct passage of ions across cell membranes in the lungs and other organs, such as chloride and sodium. In cystic fibrosis, frameshift mutations cause organ dysfunction, recurrent lung infections, and pancreatic damage.
3. Leigh Disease: The NADH dehydrogenase (ubiquinone) Fe-S protein 4 (NDUFS4) gene has a frameshift mutation that makes Leigh illness. Leigh illness. Leigh disease is a mitochondrial mutational illness that manifests as a progressive neurodegenerative condition that begins in childhood. The patient had feeding problems, hypotonia, seizures, central respiratory impairment, and failure to thrive in this case.
4. Type A Niemann-Pick Disease: Type A Niemann-Pick disease has been linked to a frameshift mutation in the acid sphingomyelinase gene (fsP330).
5. Crohn’s Disease: A frameshift mutation in the NOD2 gene causes Crohn’s disease susceptibility. The truncated protein NOD2 is produced by cytosine insertion (3020insC), which has been linked to Crohn’s disease.
6. Specific Diseases: frameshift mutations can cause malignancies including lung cancer, colorectal cancer, and hereditary breast, ovarian, and pancreatic cancer.
7. Hypertrophic Cardiomyopathy: One of the main causes of sudden death in young adults is hypertrophic cardiomyopathy. Hypertrophic cardiomyopathy is a cardiac myocyte genetic disease. A frameshift mutation in Troponin C induces hypertrophic cardiomyopathy (c.363dupG or p.Gln122AlafsX30).
8. Smith–Magenis Syndrome: caused by an interstitial deletion in the retinoic acid-induced 1 (RAI1) gene. This is an uncommon multiple congenital abnormality or mental retardation condition. Mental retardation, craniofacial and skeletal abnormalities, speech and developmental delays, unique behavioural characteristics, and sleep disruption are all common in these people.
9. Hereditary Polyneuropathy: a dominant-negative frameshift mutation in the LRSAM1 gene causes hereditary polyneuropathy.
Effects of Frameshift Mutations
Frameshift mutations can be advantageous, harmful, or fatal. Induction of frameshift mutation, for example, has been utilised to produce bacteria capable of generating nylonase, a degrading enzyme. Some incidences of albinism have been linked to the premature termination of any of the enzymes required for melanin synthesis.
Various mutations, including frameshift mutations, in the HEXA gene, which codes for the alpha subunit of the lysosomal enzyme beta-N-acetylhexosaminidase A, cause Tay Sachs illness.
Point Mutations vs Frameshift Mutations
Let’s compare and contrast point mutation and frameshift mutation to see how they vary. In point mutation, one base in the nucleotide sequence is replaced by another base. As a result, the nucleotide sequence or nucleic acid reading frame stays unaltered.
Point mutation is also known as single base substitution because of this. Point mutations are divided into two categories: transition and transversion.
Purines and pyrimidines make up DNA. When a purine base is substituted for another purine base, transition point mutation occurs, whereas transversion happens when a pyrimidine or vice versa is exchanged for a purine base.
The insertion or deletion of a base in a frameshift mutation leads to a change in the nucleotide’s reading frame in a nucleic acid.
Frameshift Mutation Citations
- Frameshift mutation, microsatellites and mismatch repair. Mutat Res . 1999 Nov;437(3):195-203.
- Frameshift mutation: determinants of specificity. Annu Rev Genet . 1990;24:189-213.
- Frameshift mutation of UVRAG: Switching a tumor suppressor to an oncogene in colorectal cancer. Autophagy . 2015;11(10):1939-40.
Share
Similar Post:
-
Meiosis: Definition, Stages, and Examples
Continue ReadingMeiosis Definition
Meiosis is defined as the process of cell division that results in the formation of a haploid “daughter” cell with the same haploid chromosomal number as the diploid “parent” (“original”) cell. After meiosis, the haploid cell would have just one portion of the parent cell’s multiple homologous chromosomal pairs.
What is Meiosis?
Meiosis is important because it decreases the number of chromosomes to half and allows for genetic diversity through genetic recombination and independent assortment.
Meiosis creates four haploid cells that may grow into potential gametes, resulting in a new individual with the complete number of genes when fertilisation occurs, preserving chromosomal number integrity over generations while increasing genetic variety and variability in population forms.
Meiosis comes from the Greek term meioun, which means “to diminish” (less).
The generation of gametes (egg cells or sperm cells) or spores is the primary purpose of meiotic division. The meiosis process occurs in the human body to reduce the number of chromosomes in a normal cell (46 chromosomes) to 23 chromosomes in eggs and sperm. As a result, the number of chromosomes in meiosis is cut in half.
As a result, when the two haploid cells merge during fertilisation, the number of chromosomes in the generated cell is restored as somatic cells (each with 46 chromosomes).
Meiosis is split into two stages: meiosis 1 and meiosis 2. Each component contains four stages (prophase, metaphase, anaphase, and telophase), which are identical to the four phases of mitosis.
The most complex portion of meiosis (i.e., meiosis I) is the initial part of the meiotic division. Because it comprises five substages: leptotene, zygotene, pachytene, diplotene, and diakinesis, prophase I takes roughly half the time it takes for meiosis. The chromosomes’ activity and structure change at each step, providing insight into prophase I’s intricacy.
Three major characteristics distinguish meiosis from mitosis:
1. Reverse recombination occurs during meiosis I. (also known as chiasma development or crossing across)
2. Meiosis I is characterised by the pairing of homologous chromosomes.
3. In Meiosis I, the cohesive sister chromatids are released in a two-step procedure.
On the mitotic spindle, these characteristics enable homologous segregation. The two sister homologous chromosomes separate during meiosis II. For the diverse events occurring in each meiosis stage, these varied chromosomal behaviours are detailed below. It’s also worth noting that these events are intertwined.
This indicates that distinct processes during chromosomal pairing, including reciprocal recombination, crossing-over, and chiasma formation, are linked. Hence, the only effective recombination mechanism during meiosis I prophase will be the one that provides proper homologous chromosome segregation at meiosis I.
The initial number of chromosomes is halved after the two subsequent chromosomal divisions. Meiosis I begins when the S phase is completed and the replication of the parent chromosome produces identical chromatids.
The chromosomes begin to couple together and eventually partition into two distinct cells. The chromatids, on the other hand, stay intact, and at the conclusion of meiosis I, each of the newly produced daughter cells will have one of the homologous chromosomes with two chromatids.
Meiosis II occurs after Meiosis I. After that, the two chromatids will split and form two daughter cells. As a result, four daughter haploid cells are formed at the end of meiosis II, each carrying one copy of each chromosome.
The pairing of homologous chromosomes after DNA replication not only allows for the segregation of meiotic chromosomes but also aids in the recombination of maternal and paternal chromosomes. This chromosomal pairing takes place during the prophase of meiosis I.
The first meiotic division (or meiosis I) and the second meiotic division (or meiosis II) are the two nuclear divisions that occur during meiosis (or meiosis II). The 4 key phases of meiosis are prophase, metaphase, anaphase, and telophase. They are also classified as I or II depending on whether they occur in meiosis I or II.
Meiosis Function
Consider this: if gametes (eggs and sperm) were generated only by mitotic division, rather than meiosis, the gametes would have the same number of chromosomes as diploid somatic cells. As a result, when the gametes unite during fertilisation, the resultant zygote will have four homologous chromosomal sets and will be tetraploid.
This “doubled chromosome content” situation will be passed down to future generations, resulting in chromosomal abnormalities. Throughout generations, the chromosomal number has fragmented and unkept. This is a chromosomal anomaly, to be sure.
Gametes are generated by the process of meiosis to keep the number of chromosomes constant in each generation. During the production of gametes, meiotic cell division reduces the number of chromosomes to haploid.
One cycle of chromosomal DNA replication precedes two rounds of nuclear division in meiosis. As a result of the parent cell’s meiotic division, four daughter nuclei (each of which is present in a new daughter cell) are generated.
Only a haploid number of chromosomes are found in each daughter cell nucleus. Gametes haploid cells are formed in two rounds: Meiosis I and II, with just one cycle of DNA replication (at the S phase of interphase).
Meiosis vs Mitosis
The two primary types of cell division are meiosis and mitosis. The distinctions between them are in mitosis only one nuclear division takes place and in meiosis two divisions. Meiosis produces haploid cell and they are sex cells, whereas in mitosis diploid cell are formed and are the somatic cells.
The end-product of mitosis is two daughter cells and in mitosis four daughter cells. Asexual reproduction takes place in mitosis and sexual reproduction in meiosis, thus crossing over takes place whereas in mitosis no crossing over is seen.
Phases of Meiosis
Meiosis is the process through which a parent diploid cell divides into four haploid cells. There really are two main phases of meiosis: meiosis I and meiosis II. Meiosis 1 is the first stage of meiotic division, often known as the reduction division of meiosis. This is due to the fact that the number of chromosomes is cut in half at this stage, resulting in the creation of haploid chromosomes.
In a process or occurrence known as a synapse, each pair of chromosomes comes close together to exchange a portion of their genetic material. This happens in the early phases of meiosis 1, especially during prophase I.
The homologous pairs of chromosomes come very close together and bond closely to each other during prophase 1 of meiosis I, and they virtually behave as one single unit. This unit is known as a bivalent or tetrad (indicating that each chromosome consists of two sister chromatids, so the sum of bivalent is four chromatids).
After aligning at the spindle equator, the bivalent separates into two pieces, allowing each chromosome to travel to the spindle pole on the opposing side. As a result, following meiosis I, each newly formed daughter nucleus is haploid, as it only carries one bivalent chromosome.
Meiosis II, the second stage of the meiosis cell cycle, is similar to mitosis in that it results in the formation of two daughter cells when the two chromatids separate.
As a result, meiosis I is the stage during which the meiosis cycle’s distinctive activities take place. Nonetheless, each step of the meiotic division, such as prophase, metaphase, anaphase, and telophase, is split in a manner that mimics the mitotic division.
The prophase of the first meiotic division, on the other hand, is far more complex and time-consuming than the prophase of mitosis. The prophase of the second meiotic division, on the other hand, is simpler and shorter.
Meiosis in a Nutshell
A cell’s chromosomes are replicated before it enters meiosis (during the interphase). The chromosomes condense along the nucleus’s centre during meiosis I and pair with their homologues during crossing over. The chromosomal pairs then split and travel to opposing ends of the cell.
For the first time, the cell splits into two cells. Both cells will go through meiosis II, when they will divide into two cells, each carrying one of each detached chromosome’s sister strands (chromatids), resulting in four genetically distinct haploid cells.
Meiosis Satges
Meiosis I occurs after interphase, when the chromosomes duplicate during the S phase of the cell cycle. During the early stages of prophase, I, the chromosomes condense. The cell’s two centrosomes go to the cell’s two opposing poles to prepare it for nuclear division.
Pairs of chromatids make up homologous chromosomes. After pairing, these chromosomes form bivalents in order to align at the spindle equator during metaphase I.
During anaphase, homologous chromosomes are separated from one another, but the two sister chromatids remain connected.
i. Prophase I
The most difficult phase of meiosis I is prophase I, which is split into five stages: leptotene, zygotene, pachytene, diplotene, and diakinesis.
The chromatin fibres condense into thread-like fibres at the Leptotene stage, resembling the established structure at the onset of mitosis.
The fibres are more condensed at the zygotene stage, allowing them to be recognised as distinct chromosomes. The bivalents arise when the pairs of chromosomes get firmly linked together as a result of synapsis.

The development of bivalent is crucial in the process of crossing over, which involves the exchange of DNA segments holding genetic material between two nearby chromosomes. During the pachytene stage, this procedure occurs. For gene recombination, the matching regions of chromosomes exchange genetic information.
During the pachytene stage, chromosomes are compressed to roughly a fifth of their original length. The two chromosomes of each bivalent split from one another at the centrosome during the diplotene stage. Chiasmata, which are links found when two homologous chromosomes swap DNA segments, keep the two chromosomes together.
Diplotene is characterised by the resumption of transcription, the de-condensation of chromosomes, and the temporary halt of meiosis. When the chromosomes are re-condensed to their maximal level of compaction at the start of prophase I’s last step, diakinesis, the centrosomes move even faster.
Only the chiasmata keep the chromosomes together. Spindles develop, nucleoli vanish, and the nuclear envelope vanishes at this point. Meiosis prophase 1 ends and meiosis metaphase 1 begins when the meiotic spindle begins to develop and the nucleoli begin to disintegrate.
ii. Metaphase I
After attaching to the microtubules with their kinetochores, the bivalents migrate to the equator of the spindle during this phase. This bivalent migration to the cell’s equator generally occurs only during meiosis I and not during mitotic division.
Each bivalent includes four chromatids, which means each bivalent also contains four kinetochores. These kinetochores appear to be in close proximity to one another, as though they are a single unit facing the same cell pole.

Each kinetochore can be attached to the microtubules of the spindle pole on the opposite side using this configuration. This arrangement is the initial step in preparing the chromosomes for separation during the anaphase that follows.
The paternal and maternal chromosomes are aligned on one pole of the cell at this time, and each newly created daughter cell will get a combination of paternal and maternal chromosomes during their migration to the opposing poles during anaphase.
iii. Anaphase I
The movement of homologous chromosomes to the spindle poles with the help of their kinetochore is the first step in anaphase. One of the key distinctions between meiosis and mitosis is this stage.
During mitosis, the sister chromatids are pushed to opposing poles, causing them to split. The two sister chromatids stay connected during meiosis, and following separation, the homologous chromosomes migrate toward the spindle poles.
As a result, by the end of meiotic anaphase I, each spindle pole contains a haploid number of chromosomes. During mitosis, chromatid separation is accomplished by cleaving the two sister chromatids using an active enzyme called separase.
To counteract separase’s activity during meiosis, the cell generates shugoshin, a protein that inhibits chromatid separation by shielding the centrosomal region of the chromosome where the cleavage occurs.
iv. Telophase I
Telophase 1 is the last phase of meiosis I, and it is marked by the movement of chromosomes to the spindle poles. Before cytokinesis, a nuclear membrane might have been created around chromosomes, resulting in two daughter cells with haploid sets of chromosomes. After the start of meiosis II, the chromosomes usually condense.
Results of Meiosis I
By the completion of meiosis, I, cytokinesis has aided in the development of two haploid nuclei cells. Each haploid cell’s chromosomes will be made up of two chromatids joined at the centromere.
Meiosis II Stages
Interphase meiosis occurs between the conclusion of meiosis I and the start of meiosis II. This stage is not connected with DNA replication since each chromosome already has two chromatids that have previously been replicated by the DNA synthesis process before the start of meiosis I.
In a nutshell, DNA is duplicated once before meiosis begins. Meiosis II, also known as second mitotic division, serves a similar goal as mitosis in that two new chromatids are orientated in two new daughter cells. As a result, the second meiotic division is also known as the meiotic division of separation.
i. Prophase II
Prophase II is the stage that occurs after meiosis I or interkinesis, and it is marked by the breakdown of the nuclear envelope and nucleolus, as well as the thickness and shortening of the chromatids, and centrosome replication and migration to the polar side. Prophase II is less complicated and shorter than prophase I, and it resembles the mitotic prophase in appearance.
Prophase II, on the other hand, differs from prophase I in that chromosomal crossing occurs only during prophase I and not during prophase II. At the completion of prophase II, metaphase II begins.
ii. Metaphase II
Metaphase II of meiotic division is identical to metaphase II of mitotic division, except that metaphase II has half the number of chromosomes and is distinguished by chromosomal alignment in the cell’s centre.
iii. Anaphase II
It is the stage after metaphase II, during which the sister chromatids split and migrate towards the cell poles. Anaphase II is similar to mitotic anaphase in that both involve chromatid separation. The shortening of the kinetochore causes sister chromatids to migrate to the cell’s two ends.
iv. Telophase II
Telophase II is the final stage of meiosis, in which four haploid cells are generated from the two cells produced during meiosis I. The newly formed cells’ nuclear membranes are fully established, and the cells are entirely separated at the conclusion of this phase.
However, sperm in humans and other animals are not completely functional at the conclusion of telophase II because they require the development of flagella to operate correctly.
Results of Meiosis II
After telophase II and cytokinesis, four haploid cells are formed, each of which has just one of the two homologous pairs of chromosomes. The genetic information from the maternal and paternal chromosomes is mixed in the haploid cells formed. These cells have a role in both the genetic variety of individuals within the same species and the evolution of animals.
Meiosis Examples
Meiosis is found in the life cycles of many creatures, including fungi, plants, algae, animals, and humans.
Meiosis can generate spores or gametes depending on the species, with gametes (sperm cells and egg cells) being produced in humans and other animals, while spores are produced in plants and algae.
Meiosis in Humans and Other Animals
In humans and other animals, meiosis generates haploid gametes. It is an important aspect of gametogenesis. Gametogenesis is the biological process of producing gametes, as the name suggests.
There are two types of gametogenesis in humans and other animals: spermatogenesis (the creation of male gametes, such as sperm cells) and oogenesis (the formation of female gametes, such as eggs) (formation of the female gamete, i.e., ovum or egg cell).

A diploid oocyte produces four haploid gamete cells during oogenesis. Only one cell survives to become an egg, while the other three become polar bodies.
This effect is caused by the oocyte’s uneven division during meiosis, in which one of the produced cells obtains the majority of the parent cell’s cytoplasm while the other generated cells degenerate, resulting in an increase in the concentration of nutrients in the formed egg. During prophase I of meiosis, the egg cell develops the majority of its specialised activities.

After meiosis and post-meiotic processes, such as spermiogenesis, when the sperm cell grows by obtaining a functioning flagellum and discarding much of its cytoplasm to form a compacted head, the sperm gets its specialised characteristics in order to develop into a functional gamete.
Meiosis is a process that happens throughout an organism’s reproductive period. However, in humans, meiotic division happens at various times. For example, in males, it begins during puberty and continues throughout their lives.
Females’ main oocytes will be stopped at prophase I by the time they reach adolescence, and they will proceed through the next phases of meiosis. However, each primary oocyte will cease at metaphase II of meiosis II when it develops into a secondary oocyte at ovulation.
Meiosis will only continue and finish during conception. Meiosis will stop if the secondary oocyte is not fertilised, and the arrested secondary oocyte will dissolve. Menstruation will start soon.
Meiosis in Plants and Algae
Plants and algae are multicellular creatures that produce both haploid and diploid cells throughout their lives. The haploid spores are generated by meiosis in this phenomenon known as alternation of generations. In plants and algae, this is also known as sporic meiosis.
The produced spores germinate and proceed through mitotic division, resulting in a haploid plant or algae. Because gametes are generated by mitotic division of already existing haploid cells, the haploid form is referred to as a gametophyte.
During fertilisation, the gametes unite to generate the diploid type of cells. Meiosis creates the spores from the diploid form. As a result, the diploid form is referred to as the sporophyte.
Meiosis in Fungi
In their life cycle, fungi have both asexual and sexual stages. The mycelium, for instance, may go through both sexual and asexual phases.
When haploid mycelia reach the sexual phase, they undergo plasmogamy (the union of two protoplasts) and karyogamy (the fusion of two protoplasts) (the fusion of two haploid nuclei). The development of the diploid zygote occurs after karyogamy.
The zygote develops into a stalked sporangium, which via meiosis produces haploid spores. Meiospores are the spores generated by meiosis, as opposed to mitospores, which are formed by mitosis. The sporangium will produce haploid spores (reproductive cells), each of which will germinate into a new mycelium.
Thus, in fungi, meiosis is the third step in the sexual phase’s sequential phases, which begin with plasmogamy and end with karyogamy. Meiosis is necessary for the fungus to return to its haploid stage.
Errors in Meiosis
Meiosis is prone to mistakes, which might have an impact on a person’s capacity to reproduce. Human longevity is severely harmed by abnormal meiosis. Infertility and the production of genetically unbalanced gametes can both be caused by errors in the meiosis stages.
Meiotic errors are the primary cause of congenital malformations as well as mental abnormalities in new born infants caused by genetic damage.
In more than 30% of human oocyte pachytene, errors in chromosomal pairing and recombination are present, resulting in a condition known as asynapsis, in which homologous chromosome pairing fails.
Failure of chromosomal pairing in yeast can cause cell death by triggering the cell’s checkpoints. A similar phenomenon may be seen in human germ cells. As a result of the rise in oocytes with chromosomal pairing faults, the number of germ cells will be depleted, resulting in early menopause in women.
Infertility results from mistakes in the phases of meiosis of spermatocyte development, as the quantity of functional sperm generated decreases.
Because a man generates around 300-400 million sperm each day, but a woman produces about 300-400 oocytes over her lifetime, depletion in the number of germ cells is more substantial in females than in males.
When chromosomal pairs fail to cross over properly during metaphase I, the unpaired chromosomes segregate randomly, increasing the chance of producing aneuploid gametes with an unbalanced number of chromosome copies. Furthermore, due to unsuccessful crossing-over, spermatocytes may be destroyed by apoptosis or necrosis.
Biological Importance of Meiosis
Because the balance between the number of chromosomes that are doubled during fertilisation and the halving of chromosomes during gamete production is maintained, meiosis and mitosis are two critical phases of the cell cycle for any organ that reproduces sexually.
A sexually reproducing organism’s cell cycle is divided into two distinct phases: haploid and diploid.
The haploid phase of meiosis begins with gamete creation and concludes with the development of a zygote during fertilisation, whereas the diploid phase begins with the formation of a zygote by the fusing of two gametes and terminates with meiotic cell division during gamete formation.
To complete the life cycle of sexually reproduced creatures such as humans and animals, meiotic division creates four haploid cells from one diploid cell.
The chromosomal DNA doubles in the parent diploid cell before meiosis, and four haploid nuclei are produced as a result of two subsequent diploid nucleus divisions.
Meiosis is physiologically significant since it is responsible for sexually reproduced organisms’ genetic variety, when the chromatids of two homologous chromosomes synapse and exchange portions of their genetic content during prophase I.
Meiosis is important because it decreases the number of chromosomes by half and allows for genetic diversity through genetic recombination and independent assortment.
Meiosis creates four haploid cells that may grow into potential gametes when fertilisation occurs, resulting in a new individual with the complete number of genes when fertilisation occurs, preserving chromosomal number integrity over generations while increasing genetic variety and variability in population forms.
Meiosis Citations
Share
Similar Post:
-

Homogeneous: Definition, Types, and Examples
Continue ReadingHomogeneous Definition
Homogeneous can be defined as “the same” or “similar.” It can be used to describe things that have similar characteristics. Homogeneous substances, for example, are substances that are homogeneous in volume and composition across their whole volume. As a result, two samples obtained from two different portions of homogeneous mixtures and substances will have the same compositions and properties.
Homogeneous Etymology
The word homogeneous is derived from two Greek words: “homo” (meaning “the same”) and “genous” (meaning “kind”). As a result, homogenous refers to individuals who are all perceived to be the same, similar, or present in the same proportion.
What is Homogeneous Mixture?
Homogenous means “of the same sort” or “similar.” It’s the ancient name for homologous in biology, which means “having matching components, similar structures, or the same anatomical locations.” Homogenous is derived from the Latin homo, which means “same,” and “genous,” which means “kind.” homogenous is a variant. Heterogeneous is the antonym of homogeneous.
A mixture is formed when two or more components combine without undergoing any chemical changes. The mechanical blending or mixing of objects like elements and compounds defines a mixture. There is no chemical bonding or chemical change in this process.
As a result, the chemical characteristics and structure of the components in a combination are preserved. Size, form, colour, height, weight, distribution, texture, temperature, radioactivity, structure, and a variety of other characteristics all stay consistent throughout the homogeneous material.
When a pigment (such as ink) is combined with water, the resultant solution is highly homogeneous, which is a fairly common example of homogeneous in our daily lives. The colour combines equally with water, and any area of the solution has the same makeup.
Mechanical techniques can be used to separate them. Centrifugation, filtration, heat, and gravity sorting are some of the methods.
That’s all there is to it when it comes to the term’s use in chemistry or biology. The term “homogenous” is used in various research areas, such as ecology, to describe a population’s homogeneity.
A group of humans raised only by asexual reproduction – with identical genes and traits — is homogeneous, for example. Scientists hypothesized that if various orientations came from the same source, the cosmos would behave similarly. Evolutionary biology is another area of biology where the term homogeneous is employed.
Homogeneous is an ancient word for homologous, which refers to anatomical components that exhibit structural similarities, such as those generated by descent from a common ancestor.
The term homogeneous has been used widely in different fields of research, such as biology, chemistry, and ecology, but it is always used to describe organisms in a mixture who have the same properties.
In chemistry, homogeneous refers to a combination in which the ingredients are uniformly distributed. However, there are no chemical connections between them at the molecular level. Air is the most typical example of a homogeneous mixture in our environment.
Homogenous vs Heterogenous
A mixture, as previously stated, is the physical coming together of components (which, in chemistry, can be elements or compounds). There are two sorts of mixtures: homogeneous and heterogeneous.
The opposite of homogeneous is heterogenous (variant: heterogeneous). It refers to the components in a combination that have distinct properties (“hetero,” which means “different”). The most obvious example of a heterogeneous combination is oil and water, which form two distinct layers that are immiscible with each other, resulting in two distinct layers.
One of the most notable characteristics of heterogeneous mixes is that the particles are not dispersed equally throughout the mixture. Analysing the combination with the naked eye reveals the heterogeneous character of the mixture. In addition, the components of all heterogeneous mixes are not uniform.
Composition is similar in homogenous mixtures and dissimilar in heterogenous. In heterogenous mixtures, various phases are seen and single phase is seen in homogenous mixtures.
Substance can be sorted from each other by physical methods such as distillation, evaporation, centrifugation, chromatography, crystallization in both types of mixtures. Variation and a smaller number of species exist in homogenous mixtures, and the reverse is seen in heterogenous mixtures.
Although the concepts and compositions of homogeneous and heterogeneous substances are vastly different, both are prone to change depending on context and composition. Let’s take the example of blood. If we look at the blood with our naked eyes, it seems to be homogeneous.
Blood, on the other hand, has a variety of components under the microscope, including red blood cells, plasma, and platelets, showing that it is heterogeneous.
Homogeneous Examples
We come across numerous examples of homogeneous mixes and entities in our daily lives. In biology, a homogeneous population is one in which all of the individuals have virtually the same genetic makeup, as a result of some types of asexual reproduction.
Asexual reproduction produces homogeneous children who are identical to each other, including their parents.
Many animals, such as goat populations, look homogenous but are not because they reproduce through sexual reproduction.
According to experts, homogeneity reduces biodiversity, and as a result, the odds of early extinction due to environmental changes are significant. Animal cloning is a frequent example of a homogeneous population.
Dolly the sheep was the first mammal to be successfully cloned from a somatic cell in an adult.
Homogeneous species are those that exhibit indistinguishable characteristics and appear to be identical. Such species appear to have a lower level of biodiversity.
The diversity and frequency of species in a particular region and period, as well as the ecosystem’s homogeneity, may be quantified using a specific fundamental unit called species richness.
Species richness refers to the number of different species found in a specific ecological community. It displays the relative abundance of species rather than the total number of species in the environment. As a result, in a homogeneous environment, species richness will be lower, as high species richness indicates variability.
This is particularly evident in endemic species, which are species that have evolved through time in a specific geographic region and aren’t found anywhere else.
Grass, trees, ants, fungus, and certain animals are all instances of homogeneous in the ecosystem. Many endemic species found nowhere else in the world may be found in New Zealand.
Homogeneous used to be a very popular term in evolutionary biology to describe physically comparable features in various species, indicating a shared evolutionary origin.
The anatomical characteristics of several animal forelimbs are depicted. A similar evolutionary ancestor is shown by the identical forelimb bone components.
Homogeneous Summary
As a result of the preceding discussion, homogeneous substances are those that are uniform in volume and composition throughout. Homogeneous mixtures in chemistry have the same size, shape, colour, texture, and many other characteristics.
A solution that does not separate from each other over time is known as a homogeneous mixture. Homogeneous species are those that are genetically similar but lack biodiversity and species richness, as defined in biology and ecology.
Similarly, various solutions are widely used in our daily lives, and the blood and DNA in our bodies are both homogeneous. Heterogeneous mixes have properties that are the polar opposite of homogeneous mixtures.
As a result, the heterogeneous mixture contains non-uniform compositions and numerous phases that cannot be distinguished by physical changes. Furthermore, they are culturally varied and affluent.
Similarly, it has been demonstrated that both homogeneous and heterogeneous mixes are prone to change depending on their environment and composition. As a result, both heterogeneous and homogeneous mixes might be seen as equally important.
Homogeneous Citations
- Synthesis of Oxazolidin-2-ones from Unsaturated Amines with CO 2 by Using Homogeneous Catalysis. Chem Asian J . 2018 Sep 4;13(17):2292-2306.
- Recent Advances Utilized in the Recycling of Homogeneous Catalysis. Chem Rec . 2019 Sep;19(9):2022-2043.
- A review of thermal homogeneous catalytic deoxygenation reactions for valuable products. Heliyon . 2020 Feb 20;6(2):e03446.
Share
Similar Post:
-

Hyaline Cartilage: Definition, Function, and Examples
Continue ReadingWhat is Hyaline Cartilage?
Hyaline cartilage tissue is a kind of cartilage tissue that is also known as hyaline connective tissue or hyaline tissue. It’s the most prevalent kind of cartilage, with a lustrous, smooth look.
Cartilage is a tough and pliable connective tissue that protects bone ends, discs, and joints from wear and strain. At the embryonic stage, cartilage acts as the early skeletal structure in various animals, including humans.
The majority of it is replaced by bone as the animal grows. The cartilaginous skeleton of an adult cartilaginous fish (Chondrichthyes) is preserved. When compared to bone tissues, cartilage tissues are more flexible and elastic.
Around the bones of free-moving joints, hyaline cartilage is present. Articular cartilage is what this is called. The tissue present in the walls of the respiratory tract is another example of hyaline cartilage. The bronchi, nose, trachea rings, and rib tips all fall under this category.
Hyaline Cartilage Etymology
Hyaline cartilage is a kind of cartilage that has a lustrous, white, semi-transparent look with a little blueish tinge, according to biology. The name hyaline comes from the Greek word hyalos, which means “glassy,” suggesting the material’s gleaming, smooth look. The larynx, trachea, and bronchi are all places where it may be discovered.
Hyaline Cartilage Structure
Chondroblasts (or perichondrial cells) generate the extracellular matrix (or ground material), chondrocytes reside in gaps called lacunae, and collagen fibres make up cartilage.
Hyaline Cartilage Location
Mesenchymal cells, which are stem cells present in the bone marrow, give rise to it. Because it lacks blood vessels and nerves, hyaline cartilage has a relatively basic structure. It gets its nutrition from surrounding tissues via diffusion.
Hyaline cartilage has a gleaming, semi-transparent white look with a blueish tint. The name hyaline comes from the Greek word hyalos, which means “glassy,” suggesting the material’s gleaming, smooth look. Surprisingly, as the tissue matures, this look fades. Hyaline cartilage forms the initial skeleton in an embryo, which then changes as the embryo grows. Endochondral ossification is the process that causes this.
Fine type II collagen fibres, chondrocytes (matrix-producing cells), and the extracellular matrix make up hyaline cartilage (or ground substance). Collagen fibres of type II are thinner than collagen fibres of type I. Collagen types I, IV, V, VI, IX, and XI are also found in trace amounts and assist in holding the fibres together.
Glycosaminoglycans (GAGs), proteoglycans, and glycoproteins are abundant in the extracellular matrix, commonly known as the ground material. The extracellular matrix (ECM) covers the gaps between cells and fibres.
Long polysaccharides consisting of amino sugars that attract sodium and potassium ions are known as GAGs. These ions carry water with them. As a result, the amount of water in the extracellular matrix may be controlled.
Sulfated GAGs include chondroitin sulphate and keratan sulphate, whereas non-sulfated GAGs include hyaluronic acid. All of these substances can be present in cartilage’s extracellular fluid.
Proteoglycans and glycoproteins are a combination of amino acids and carbohydrates. They form a gel-like fluid that helps to absorb compression and tension by binding extracellular molecules and components together.
In hyaline cartilage, chondrocytes are the sole cartilage cells. These cells begin as chondroblasts (or perichondrial cells), which generate a cartilaginous matrix before becoming trapped inside it in tiny areas known as lacunae.
Chondrocytes are responsible for the development, repair, and maintenance of the extracellular matrix. Due to their restricted replication ability, chondrocytes have a limited healing capability. They seldom have cell-to-cell communication and are just concerned with preserving their local environment.
The perichondrium covers the hyaline cartilage in most cases. The perichondrium covers the articular cartilage on the ends of growing bones but not in adults. There are two layers to the perichondrium: an exterior layer and an interior layer.
The outer layer is fibrous cartilage that generates collagen fibres, whereas the inner layer contributes to cartilage development by producing chondroblasts or chondrocytes.
Hyaline Cartilage Examples
Hyaline cartilage is a kind of articular cartilage. It differs from normal hyaline cartilage in that the chondrocytes at the surface are flattened. It is 2 to 4 mm thick in people. There are no blood vessels, nerves, or lymphatics in it. It has a thick ECM but sparse chondrocytes.
The chondrocytes take on a more normal shape as they go deeper into the tissue. The cells are located in columns with a calcified matrix in the cartilage’s deep layers. Collagen strands create arches, which provide a robust structural arrangement that can bear pressure.
Type II collagen makes up articular cartilage, although it also contains tiny quantities of type VI, IX, X, and XI collagen.
Different zones make up the articular cartilage. The superficial zone is the first, followed by the intermediate transitional zone, the deep zone, and the calcified zone. There are three areas within each zone.
The pericellular region, territorial region, and interterritorial region are the three.
The superficial zone accounts for around 10% to 20% of the cartilage’s overall thickness. Here you’ll find collagen fibres II and IX. It has a significant number of chondrocytes with a flatter look.
The synovial fluid is in direct touch with the superficial zone, which shields the deeper layers from force and stress.
The intermediate zone runs parallel to the superficial zone and serves as a link between the two levels. This zone accounts for about 40-60% of the overall cartilage thickness.
It is made up of more dense collagen fibres and proteoglycans. The chondrocytes in this sample are spherical and in tiny numbers.
The purpose of the intermediate zone is to defend against compacting pressures. Following the intermediate zone, the deep zone gives the best resistance to compacting pressures. It has the largest proportion of proteoglycans and the least amount of water.
Collagen fibres are organised into columns and chondrocytes are positioned at right angles to the surface. It accounts for around 30% of the overall volume of articular cartilage.
Finally, the cartilage is attached to the bone via the calcified zone. It accomplishes this by attaching the deep zone collagen fibres to the subchondral bone.
Hyaline Cartilage Histology
Hyaline cartilage connective tissue is made up of cells and fibres inside an extracellular matrix, as previously stated. Hyaline cartilage histology explains the appearance of hyaline cartilage under a microscope.
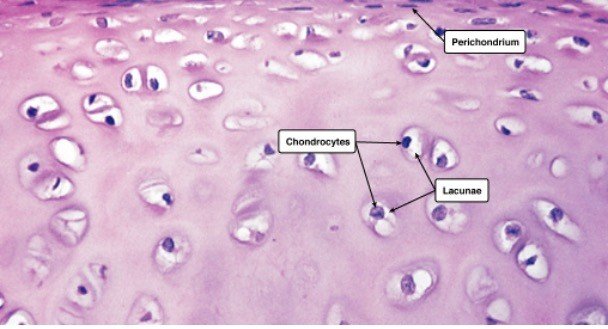
The shape of the chondrocytes might be spherical or angular. The cells in mature cartilage are found in isogenous clusters, each produced from a single progenitor cell. The matrix appears to be optically homogeneous and basophilic.
The explanation for this is that the collagen fibres are obscured by the large quantity of sulfated GAGs in the matrix. Because type II collagen fibres are so tiny, the extracellular matrix appears gleaming and smooth.
Within the extracellular matrix, there is no homogeneous distribution. As a result, the three fundamental zones are visible.
1. The capsular matrix, which is made up of a narrow zone around each lacuna. The greatest concentration of sulfated GAGs may be found here.
2. The capsular matrix is surrounded by a territorial matrix.
3. The interterritorial matrix, which is less basophilic due to a larger amount of collagen and a lower quantity of sulfated GAGs.
Under the microscope, hyaline cartilage may be examined using the hematoxylin and eosin (H & E) staining method as well as the Van Geison staining method. Picric acid and acid fuchsin are used in the Van Geison stain, which turns collagen red.
The staining grows lighter as it gets closer to the territorial matrix’s lacunae. The colour intensities are inverted in the H & E stained sections, although they have higher definition than the Van Geison stain.
The territorial matrix is black in hue, whereas the interterritorial matrix is much lighter. In the H & E technique, groups of chondrocytes may be detected surrounded by these darker regions. Because these chondrocytes come from the same progenitor, they form an isogenous group. Except for articular cartilage, the perichondrium surrounds the cartilage.
Hyaline Cartilage Function
Hyaline cartilage has a small number of fibres and offers a smooth surface for movement as well as a cushion to absorb stress at the point where the bones meet.
The major function of articular cartilage is to create a smooth surface that can resist friction and pressure caused by weight-bearing activities. It supports the softer tissues of the trachea and helps them to retain an open posture.
Hyaline cartilage’s primary purpose is to mechanically support the respiratory system, developing bones, and articular surfaces. The quality of our hyaline cartilage might deteriorate as we get older.
The number of chondrocytes in the surface layer of articular cartilage decreases as people become older, whereas the number of chondrocytes in the deeper layers rises. Additionally, as one gets older, the amount of proteoglycans in the extracellular matrix decreases.
Keratin sulphate levels are also up, whereas chondroitin sulphate levels are down. Hyaluronic acid volume increases as well. Due to its role as a shock absorber and frequent usage in daily activities, hyaline cartilage is prone to wear and strain. All of these characteristics can make hyaline cartilage more vulnerable to injury and illness than other cartilage forms.
Due to a lack of blood flow to the chondrocytes, cartilage tissues are prone to recovering slowly following an injury. This indicates that the matrix is taking a long time to develop. Furthermore, chondrocytes become trapped in lacunae and are unable to move to a damaged region. Scar tissue develops from damaged tissue.
Chondroitin sulphate, an anti-inflammatory mediator that decreases pain, plays a vital role in the extracellular matrix. According to research, its presence slows cartilage breakdown, preventing diseases like osteoarthritis.
Osteoarthritis develops when cartilage wears away, enabling the bones to rub against each other, producing sclerosis (hardening) of the subchondral bone (bone immediately under the cartilage) and inflammation of the synovial membrane, resulting in pain.
Hyaline Cartilage in Other Animals
The skeletons of animals in the Chondrichthyes class are entirely made of cartilage. Sharks and rays are excellent examples. Because cartilage is less thick than bone but yet offers strength, these creatures can move swiftly through the water without expending excessive effort.
Horseshoe crabs, snails, and cephalopods are instances of invertebrates possessing cartilage (predatory mollusks, e.g., octopus and squid). The branchial cartilage of the arthropod Atlantic horseshoe crab (Limulus polyphemus) is abundant in vacuolated chondrocytes, unlike that of any other arthropod.
Another kind of cartilage discovered in this species is endosternite cartilage. It has a higher fibrous content than vertebrate hyaline cartilage. It’s located near the ventral nerve cords and cartilage tissue of the gills.
The cranial cartilage of the octopus (a cephalopod) mimics hyaline cartilage and is one of the only hard sections of the octopus’s body. Cells move from the exterior to the core of the cartilage, causing it to expand. The cartilage of the common cuttlefish (Sepia officianalis) is fibrillar collagen. This cartilage has a development pattern similar to that of vertebrate cartilage.
The odontophore is a cartilage-formed feeding device in gastropods (snails, slugs, or whelks) that offers feeding support. The odontophore is a myoglobin-rich cartilage with a little portion of extracellular matrix and collagen around it.
Finally, cartilage supports the tentacles of feather duster worms (Sabellid polychaetes).
Types of Cartilage
There are three kinds of cartilage in the human body. The most prevalent, but also the weakest, kind of cartilage is hyaline cartilage. Fibrocartilage and elastic cartilage are the other two kinds of cartilage. The descriptions of each cartilage type may be found below.
i. Elastic Cartilage
Consider the distinctions between hyaline and elastic cartilage. Elastic cartilage (also known as yellow fibrocartilage) is a kind of cartilage that gives the body strength and elasticity.
Where can you find elastic cartilage?
The pinna, epiglottis, and laryngeal cartilage, as well as the auditory tube/eustachian tube, are all places where it can be found. Elastic cartilage provides greater elasticity while providing support. A thick network of elastin fibres can be found within it. It doesn’t offer any protection against mechanical stress or compression.
ii. Fibrocartilage
Fibrocartilage connective tissue is a fibrous tissue that is thick, flexible, and supports cartilage.
Where can you find fibrocartilage?
The intervertebral discs of the spine, the jaw, the knee, and the wrist are all places where fibrocartilage can be found. Large bundles of type I collagen may be seen in this fibrocartilage tissue. It is the most durable cartilage.
Fibrocartilage provides resistance to weight-bearing and pressure stresses. Fibrocartilage may be classified into four distinct categories.
1. Intra-articular fibrocartilage is the first group. This works as a cushion between joints that are subjected to a lot of stress and movement. The menisci of the knee are one example.
2. Connecting fibrocartilage, which is present in joints with restricted mobility, such as the intervertebral discs, is the second category.
3. Stratiform fibrocartilage is a kind of fibrocartilage that coats the bone grooves where tendons and muscles are present.
4. Finally, certain articular cavity borders are surrounded by circumferential fibrocartilage, which protects their edges. One acetabular labrum is an example (lining the hip socket).
Elastic cartilage, hyaline cartilage, and fibrocartilage are the three kinds of cartilage. Cartilage is a connective tissue defined by an extracellular matrix rich in chondroitin sulphate and chondrocytes as the cellular component. The most prevalent kind of cartilage is hyaline cartilage.
Hyaline Cartilage Summary
Overall, cartilage is a crucial structural component of the body that may be found in vertebrates and some invertebrates. It is a strong but soft tissue that supports, stretches, and strengthens the body.
Hyaline cartilage between joints demonstrates the importance of cartilage. The cartilage thins as we age, causing inflammation and bone friction.
Researchers in this field are working on studies that will help us better understand the mechanisms that contribute to these diseases and discover strategies to combat/treat/prevent them.
Hyaline Cartilage Citations
Share
Similar Post:
-

Hyperosmotic: Definition, Types, and Examples
Continue ReadingHyperosmotic Definition
The word “hyperosmotic” comes from two Greek words: “hyper,” which means “excess,” and “osmos,” which means “thrust” or “push.” A solution that exerts more thrust or pushes through a membrane is referred to as hyperosmotic.
To fully comprehend this concept, we must first comprehend that a solution is created by combining two components, namely a solute and a solvent. Sugar is the solute and water is the solvent in an aqueous sugar solution, for example.
(1) of, pertaining to, or characterised by an elevated osmotic pressure (usually higher than the physiological level);
(2) a state in which the total amount of permeable and impermeable solutes in a solution is larger than that of another solution.
What is Hyperosmotic?
In each system, the quantity of solute in a solution eventually dictates the direction of solvent flow. It is a well-known fact that a concentration difference causes the formation of a concentration gradient, which pushes the migration of molecules from a higher concentration to a lower concentration.
Osmosis is a phenomenon that happens when a concentration gradient causes the solvent (water) molecule to flow through a semi-permeable membrane.
As a result, a hyperosmotic solution is one that has a greater concentration of solute than a comparable solution. Seawater, for example, is hyperosmotic when compared to freshwater or tap water. A freshwater cell gets caught to a hyperosmotic environment when it is placed in a beaker with seawater.
The osmolarity of a solution is the number of solute molecules per volume or weight of the solution. The osmotic pressure exerted by a solution is controlled by its osmolarity. This is especially significant in biological systems when two solutions are separated by a membrane that is typically semi-permeable.
As a result, osmolarity can influence the flow of molecules in a biological system across a biological membrane. Maintaining cellular homeostasis requires the flow of molecules across the biological membrane. As a result, osmolarity is important for cellular homeostasis.
The osmolarity of human serum is kept within a narrow range of 285–295 mOsm/kg. Isotonicity refers to the fact that the majority of human body cells share a comparable osmolarity. Hypertonic and hypotonic fluids are defined as having greater or lower osmolarity than human serum, respectively.
The growth of osmotic pressure, which finally culminates in the creation of osmotic stress in a biological system, is caused by a variation in osmolarity. The pressure or push provided to the solvent molecules to prevent them from passing through the membrane is known as osmotic pressure.
It is critical to recognise at this point that tonicity and osmolarity are not the same thing and should not be confused. Isotonic solutions are not always isosmotic, and vice versa. In the same way, a hyperosmotic solution isn’t always a hypertonic solution. To comprehend this, we must first comprehend the idea of tonicity.
Only non-penetrating solutes have tonicity, which is always dependent on the comparative solution. An isotonic sucrose solution for a mammalian cell will be isotonic, whereas a hypotonic sucrose solution for a plant cell will be hypotonic.
This is because sucrose cannot permeate through a mammalian cell because it lacks transporters, but sucrose can permeate through a plant cell since transporters are present. As a result of sucrose’s non-permeability in mammalian cells, the isotonicity of isotonic sucrose solution in mammalian cells.
It’s crucial to remember that tonicity is governed only by non-penetrating solutes to grasp this. Hypotonic solutions have a lower concentration of non-penetrating solutes than hypertonic solutions. A 5 percent dextrose solution with no non-penetrating solutes is a classic example of a hypotonic solution.
Water movement occurs when a cell is put in a hyperosmotic yet hypotonic solution, such as 10% dextran. As a result, a solution might be hyperosmotic and hypotonic at the same time.
When the extracellular fluid’s osmolarity exceeds that of the intracellular fluid, the cell is said to be exposed to a hyperosmotic environment and will undergo hyperosmotic stress. When the extracellular fluid has a greater osmolarity, it causes water to flow out of the cell, causing cell shrinkage and finally dehydration.
A cell’s exposure to hyperosmotic fluid can be extremely harmful to it. Such cells will have to cope with water efflux, which will disturb a variety of cellular functions, including DNA synthesis and repair, protein translation and degradation, and mitochondrial dysfunction. The cell shrinks and the nucleus convexes as a result of the hyperosmotic state.
Apoptosis, which leads to cell death, is induced by cell shrinkage. When the extracellular fluid’s osmolarity is less than that of the intracellular fluid, the cell is said to be in a hypoosmotic environment. There will be an influx of water/solvent in such an atmosphere.
Physiological Importance of Hyperosmotic Property
The human body is very adaptable to such changes, and in order to do so, cells engage in osmo-adaptive reactions, in which they attempt to adjust to such changes and restore homeostasis. Failure to re-establish homeostasis, on the other hand, frequently leads to a sick or inflammatory state in the body.
Osmolarity imbalances may be harmful to cells and biological processes, and can even lead to illness. The kidney, in conjunction with the antidiuretic hormone arginine vasopressin (AVP) produced by the posterior pituitary, regulates osmolarity homeostasis in the human body.
The release of AVP from the pituitary gland is triggered by an increase in plasma osmolarity. AVP then works on the kidney, increasing the distal tubule’s membrane permeability and therefore increasing tubular reabsorption of water from the kidney. The kidney controls both the quantity of solute and water in the urine.
The urine output might have a low osmolarity (50 mOsm/L) or a high osmolarity (1200-1400 mOsm/L) depending on the body fluid state. When the body has an overabundance of water and the extracellular fluid has a low osmolarity, low osmolarity urine is produced. The urine is hypoosmotic in this situation.
Hyperosmotic urine, on the other hand, is formed when the body is dehydrated and the extracellular fluid has a high osmolarity. Higher osmolality in body fluids causes the pituitary to produce AVP, which promotes tubular water reabsorption from the kidney.
As a result of water reabsorption, the amount of water in the urine output is decreased, resulting in highly concentrated urine, also known as hyperosmotic urine. Osmolarity changes have also been linked to the initiation of inflammatory processes in the body.
Hypernatremia, heat stroke, diabetes, tissue burns, dehydration, asthma, cystic fibrosis, and uremia have all been linked to high extracellular fluid osmolarity. TNF, IL1, IL6, IL8, and IL18 are pro-inflammatory cytokines that have been linked to hyperosmotic stress-related diseases.
Consider the following example: The tubular fluid in the kidneys is:
1. When it’s at the start of the Henle loop, it’s iso-osmotic (to plasma).
2. When it’s at the end of the loop, it’s hyperosmotic (to plasma).
3. When it departs the loop, it becomes hypo-osmotic (to plasma).
Therapeutic Applications of Hyperosmotics
In the treatment of glaucoma, hyperosmotic drugs are utilised. Glaucoma is a vision or ophthalmic disease in which the intraocular pressure rises (IOP). A rise in IOP, along with impaired vision, is a very unpleasant condition for the patient.
Hyperosmotic drugs lower IOP by creating an osmotic gradient between the blood and intraocular fluid compartments, causing ophthalmic fluid to flow into the bloodstream. When glaucoma does not react to carbonic anhydrase inhibitors given topically or systemically, this treatment strategy is chosen. Hyperosmotic drugs, on the other hand, have a short duration of effectiveness and might cause systemic adverse effects.
IOP is raised in glaucoma due to the presence of vitreous fluid in the eye. The osmolality of the intravascular fluid rises when hyperosmotic drugs are administered (hyperosmolarity).
The ocular barrier, on the other hand, prevents these substances from penetrating into the vitreous fluid. The osmotic gradient is created as a result of this. As a result, fluid from the vitreous outflow enters the vascular fluid. As a result, the patient’s IOP is lowered due to the reduced quantity of vitreous fluid.
The use of hyperosmotic drugs in glaucoma patients has been shown to result in a 3-4 percent decrease in IOP. Molecular weight, dosage, concentration, rate of administration, route of administration, excretion rate, distribution, and ocular penetration are all parameters that influence the effectiveness of these drugs.
Glycerin, urea, isosorbide, mannitol, and other hyperosmotic are examples of hyperosmotic used in glaucoma treatment. These agents can be used topically, intravenously, or orally. However, systemic (parenteral) or oral administration of these drugs may have undesirable side effects.
Hyperosmotic drugs are often used to treat eye illnesses, such as glaucoma, as well as their dosage and possible adverse effects.
Hyperosmotic drugs are also used to improve vision in individuals with corneal edoema, where the agents produce temporary dehydration to reduce the cornea’s oedematous state. Hyperosmotic drugs are also used to treat cerebral edoema, in addition to ocular edoema.
Hyperosmotic drugs can also be used as a plasma volume expander in the treatment of hypovolemic bleeding.
An efficient plasma expander has been reported to be a combination of 7.5 percent sodium chloride and 6 percent dextran-70. This combination of hyperosmotic drugs (NaCl and dextran) has also been shown to decrease acute hypotension and head injury mortality.
The use of a hyperosmotic drug has been shown to have fast cardiovascular effects, including an increase in cardiac parameters such as arterial pressure, cardiac output, plasma volume, cardiac contraction, mean circulatory systemic pressure, and oxygen supply and consumption.
Hyperosmotic Stress in Plants
Plants, like mammals, are susceptible to physiological disturbances caused by hyperosmotic stress. Hyperosmotic stress is a common manifestation in plants as a result of hyperosmotic conditions (when the osmolarity outside is higher than the inside of the cell).
High salt concentrations in the soil, as well as dryness, are the typical culprits. When this happens, the plants respond by changing their genetic expression, producing intracellular osmolytes, and active endocytosis, as well as ion sequestration through vacuolar transport, to counteract the water outflow and subsequent decrease in cell volume.
If the severe disturbance is not corrected quickly, the plant cell may perish due to a loss of turgor pressure and the collapse of the plasma membrane.
Hyperosmotic Citations
- Hyperosmotic stress response: comparison with other cellular stresses. Pflugers Arch . 2007 May;454(2):173-85.
- Response to hyperosmotic stress. Genetics . 2012 Oct;192(2):289-318.
- Hyperosmotic phase separation: Condensates beyond inclusions, granules and organelles. J Biol Chem . Jan-Jun 2021;296:100044.
Share
Similar Post:
-

Mutagen: Definition, Types, and Examples
Continue ReadingMutagen Definition
A mutagen is a chemical or agent that alters the DNA sequence by causing DNA impairment. Mutation is the term for this change in DNA sequence.
What is a Mutagen?
A mutagen is a substance that induces mutations in living organisms. Physical, chemical, and biological mutagens are all types of mutagens.
Mutagenicity refers to a substance’s ability to cause changes in the base pairs of DNA, also known as mutation. The hereditary substance of a live cell is DNA. DNA is a polynucleotide that consists of many nucleotide units. Nitrogen-containing nucleobases (cytosine [C], guanine [G], adenine [A], or thymine [T]) are covalently bonded to sugar (typically deoxyribose) and a phosphate group to form these units. All of a cell’s genetic information is encoded in the standard arrangement of nucleic acid bases.
A mutagen alters the pattern and sequence of nucleic acid bases in DNA, resulting in alterations in the protein that results. Depending on whether the alterations occur in somatic or germline cells, they may be inheritable or non-inheritable.
UV radiation, X-rays, reactive oxygen species, alkylating agents, base analogues, transposons, and other mutagens are only a few examples.
Types of Mutations
Mutations are categorised as follows, depending on which cells are impacted by the mutagen:
i. Somatic Mutations
These are mutations that arise in a living being’s non-reproductive cells (somatic cells). Due to the body’s reparative and compensatory processes, many forms of somatic mutations may not develop to impact an individual. A somatic mutation, on the other hand, changes the cell’s cell division processes, can eventually lead to the development of malignant cells or tissue.
ii. Germ-line Mutations
These mutations can happen in gametes or the reproductive cells that make gametes or sex cells. These mutations are inherited and passed on to the following generation in all of their cells (both somatic and germ-line). Chemicals have been categorised based on their ability to cause mutations in germline cells.
1. Chemicals that are known or proved to induce germ-line heritable mutations based on epidemiological data are classified as 1A chemicals.
2. Chemicals classified as 1B induce germ-line heritable mutations based on mutagenicity studies performed on in vivo mammalian germ cells or somatic cells.
3. Chemicals classified as 2 are compounds that, based on in vitro studies or in vivo somatic cell mutagenicity or somatic cell genotoxicity testing in animals, are thought to induce germ-line heritable mutations.
Mutagens are known to enhance the frequency of mutations beyond what occurs naturally. However, it is critical to note that not every DNA impairment or damage may be classified as a mutation. During the cell-repair process, DNA polymerases remove the broken or changed parts of DNA in the vast majority of cases. Mutations induced by mutagens, on the other hand, tend to evade this cellular repair mechanism, resulting in a ‘permanent’ alteration in the DNA.
Type of DNA Mutations
1. Base Substitutions: Single-base substitutions are referred to as “point mutations.” Silent, missense, and nonsense mutations are the most frequent kinds of mutations.
a. Silent Mutations: The substitution of a nucleotide in the codon’s third position. As a result, there’s a good chance that an identical codon will be created, coding for the same amino acid sequence. As a result, there is no silent mutation due to a change in the gene sequence.
b. Missense: A base substitution that causes a gene sequence to code for different amino acids and, subsequently, a new polypeptide sequence to be generated.
c. Nonsense: This mutation is defined as a base substitution that results in the development of a gene sequence that truncates translation or a change that results in the generation of a stop codon that finally results in the formation of a non-functional protein.

2. Deletion: Deletion of one or more base pairs in DNA, as the term implies. When one or more base pairs are lost or removed from the DNA, this might cause a frameshift.

3. Insertions: Frameshifts can also be caused by the insertion of extra base pairs into the DNA. However, whether or whether the addition of base pairs occurs in multiples of three base pairs will determine the frameshift.
Any agent (physical or environmental) that can cause a genetic mutation or enhance the rate of mutation (biology definition). Silent mutations occur when a mutation occurs in the non-functional section of the DNA, whereas deadly mutations occur when a mutation occurs in the actively transcribed area of the DNA, resulting in cell death.
The biological impact of these DNA changes is dictated by the location of the change in the DNA, the time of mutation throughout the cell cycle, the length or magnitude of the change, and any previous mutations.
Molecular Mechanisms of Mutagenesis
Mutagens operate on DNA in a number of ways, with the following being some of the most prevalent mechanisms for mutagenesis:
DNA Damage: The particular sequence of purine (guanine and adenine) and pyrimidine (cytosine and thymine) bases connected by hydrogen bonding, i.e. guanine (G)/cytosine (C), that encodes all of a living being’s genetic information. DNA damage can be caused by a mutagen that changes these base sequences.
Spontaneous Damage: The production of apurinic/apyrimidinic (AP) sites is caused by spontaneous base deamination, hydrolysis of purines or pyrimidines, and the deoxyribose link. The sugar-phosphate backbone is eventually exposed to DNA strand breaking as a result of this. There are also tautomeric variants of the bases, which can lead to multiple base mispairing.
Chemical Adducts: Electron-deficient species, or extremely reactive electrophiles, are found in several chemical mutagens. These reactive mutagens can create covalently bound nucleophilic adducts inside DNA, which can stabilise the purine/pyrimidine bases’ alternate tautomeric configurations. The base pair coding will ultimately change as a result of this.
This will result in erroneous base pair information being transcribed, as well as increased vulnerability to DNA hydrolysis and the creation of AP sites. Additionally, some mutagens may increase DNA inter- and intrastrand cross-linking, which prevents DNA strand separation. As a result, difficulties arise during the replication, transcription, and repair of DNA.
Oxidative Damage: Mutagens can cause oxidative stress, which causes free radical production (oxygen or nitrogen). These free radicals might be hydroperoxide, hydroxyl, or superoxide moiety, and are extremely reactive molecules with unpaired electrons. These free radicals interact with DNA, causing DNA strands to break, bases to hydrolyze, or lesions to form on the DNA strand.
DNA Intercalation: Some mutagens bind to the complementary strands of DNA and intercalate between them. The hydrogen bonding between the base pairs is disrupted as a result of this. Intercalation like this eventually leads to misread or erroneous replication and transcription processes.
Metabolic Activation: The normal metabolic process, which takes place mostly in the liver, is divided into two stages: stage I and stage II. These metabolic processes seek to increase the solubility of unwanted chemicals in order to remove them from the body. The hydroxylation process takes place in Stage I, which is carried out by the cytochrome P450 enzyme system.
In Stage II, polar groups are added by the use of glutathione S-transferase, glucuronide transferase, microsomal epoxide hydrase, or acetyltransferase. The bulk of mutagens are inactivated during stage II, although some enzymes, such as flavin monooxygenase and prostaglandin H synthase, have been shown to activate mutagens.
Types and Examples of Mutagens
Mutagens can be divided into three groups;
i. Physical Agents
a. Radiation
Radiation was the first agent to be identified as mutagenic. UV rays, X-rays, alpha rays, and neutrons, among other ionising and non-ionizing radiation, have been discovered to be mutagenic. The majority of these radiations cause fatal (i.e., cell death) or sub-lethal (i.e., cell dysfunction) effects by directly destroying DNA or nucleotides via:
1. inducing DNA or protein cross-linking
2. chromosomes are broken
3. strand breakage or chromosomal loss
4. base deletion/DNA strand breaks at a molecular level
Ionizing or high-energy radiation, such as X-rays and-rays, works primarily on dividing cells and damages more than just DNA. Their impact also affects lipids and proteins. These rays produce free radical molecules that damage DNA or chromosomes.
X-rays with a dosage of 350-500 rems are deemed deadly because they cause phosphodiester links to break, causing DNA strands to break. UV rays, unlike X-rays, are non-ionizing radiation because they contain less energy. Sterilization and decontamination processes frequently use them.
UV rays cause mutagenesis through a variety of processes, including base deletion, strand breaking, cross-linking, and the creation of nucleotide dimers.
UV A, UV B, and UV C are the three kinds of UV radiation. UV-A is a kind of UV radiation with a wavelength of 320nm (near-visible range) that is known to cause pyrimidine dimerization. This kind of pyrimidine dimerization causes a change in the DNA structure, which prevents the replication fork from forming during the replication process.
Dimerization may cause health problems. UV-B, which has a wavelength of 290-320nm and is particularly deadly to DNA, has a wavelength of 290-320nm. UV-C radiation, which has a wavelength of 180-290nm, is the most damaging and carcinogenic. The ozone layer absorbs the majority of UV-C light.
b. Heat
Temperatures exceeding 95°C are dangerous for DNA. The phosphodiester bonds in DNA break at 95°C, causing the DNA strand to break. As a result, heat causes DNA to break.
ii. Chemical Agents
a. Base Analogues
They are agents that are structurally identical to bases, such as purines and pyrimidines. 5-Bromouracil and aminopurine are the most frequent base analogues that are considered chemical mutagens. Base analogues are integrated into the DNA structure during replication due to structural similarities between these agents and DNA bases.
Aminopurine is identical to adenine and may couple with C or T to create a base pair (though base pairing with C is rare). Tautomeric variants of 5-bromouracil exist. Each tautomeric molecule binds to a distinct base pair. The keto form of 5-bromouracil substitutes thymine in DNA and creates a base pair with adenine, whereas the enol tautomeric produces a base pair with guanine.
As a result, 5-bromo uracil causes a point mutation. As a result, DNA containing 5-Bromouracil switches base pairs from A-T to G-C or from a G-C to an A-T during replication, depending on the tautomeric form.
b. Intercalating Agents
They are compounds with a hydrophobic heterocyclic ring structure that closely resembles the ring structure of base pairs. These agents embed themselves in the DNA helix, causing mutations, most often frameshift mutations, by interfering with replication, translation, and transcription.
Intercalating chemicals include ethidium bromide, proflavine, acridine orange, actinomycin D, or daunorubicin, among others. Daunorubicin, Epirubicin, Epirubicin, and Mitoxantrone are some of the most commonly used anti-cancer or anti-antineoplastic medicines.
c. Metal Ions
Mineral ions such as nickel, chromium, cobalt, cadmium, arsenic, chromium, and iron produce reactive oxygen species (ROS) that induce DNA hypermethylation, increasing DNA damage and obstructing DNA repair.
d. Alkylating Agents
These chemicals cause DNA damage by causing alkyl groups to form in the DNA. The insertion of alkyl groups causes an increase in ionisation, which causes base-pairing mistakes and eventually holes in the DNA strand. Ethylnitrosourea, mustard gas, vinyl chloride, Methylhydrazine, Busulfan, Carmustine, Lomustine, Dimethyl sulphate, Temozolomide, Dacarbazine, Ethyl ethane sulphate, and Thio-TEPA are some of the most frequent alkylating agents.
These chemicals can be eliminated from the DNA during the DNA repair process via the depurination procedure. Depurination is a procedure that is not mutagenic.
iii. Biological Agents
a. Transposons and Insertion Sequences
They are DNA units that perform self-directed DNA fragment displacement and multiplication. Insertion sequences, or IS, are the simplest form of transposons (10-50 base pairs long). IS and transposons are both referred to as jumping genes because they travel across DNA.
The introduction of transposons into chromosomal DNA causes the genes’ functioning to be disrupted. IS and transposons both include information for the enzyme transposases, which aids in the creation of new transposition sites in DNA.
There are three types of transposons that are commonly found:
1. Replicative Transposons: These are transposons that keep the original locus but replicate it.
2. Conservative Transposons: The original transposon translocates itself into these transposons.
3. Retrotransposons Transpose: These transposons go via RNA intermediates to reach their destination.
b. Viruses
The insertion of viral DNA into the genome may cause genetic function to be disrupted. The Rous sarcoma virus, for example, has been known to cause cancer. Viruses can therefore be regarded as mutagenic.
c. Bacteria
Bacteria that cause inflammation, such as Helicobacter pylori, create reactive oxygen species, which cause DNA damage and a reduction in DNA repair. This raises the likelihood of a mutation.
Mutagen vs Carcinogen
It’s critical to know the distinction between mutagens and carcinogens at this point. These two words are often used interchangeably since they are so closely related. These are, however, two distinct words.
Mutagens are substances that cause heritable or non-heritable changes in DNA.
Carcinogens are substances that cause uncontrolled cell division, resulting in the formation of tumours that may or may not be malignant.
Though both words are commonly linked, mutagenic chemicals can cause changes in a cell’s genetic material, which can lead to carcinogenesis. As a result, carcinogenesis might be a side effect of mutagen-induced mutagenic alterations.
Mutagens have also been linked to an increase in the frequency of carcinogenic occurrences.
It’s also crucial to remember that while certain mutations aren’t life-threatening, carcinogenesis produces life-threatening consequences.
Teratogens is a word that is commonly used interchangeably with teratogens. Teratogens are substances that can cause abnormalities or birth defects in a foetus while having no impact on the mother. Teratogenic chemicals include ethanol, mercury compounds, phenol, lead compounds, carbon disulfide, xylene, and toluene.
Thalidomide is the most well-known teratogenic medication, which was first administered to pregnant women in the early 1950s for morning sickness. Thalidomide caused extensive congenital abnormalities such as missing legs, limbs, and ears, among other things. As a result of these numerous incidents, the medication was withdrawn from use.
Thalidomid was reintroduced for the treatment of myeloma and leprosy in the early 2000s. Phenobarbital, Valproic acid, Tretinoin, Tetracyclines, fluoroquinolones, warfarin, Propylthiouracil (PTU), methimazole (MMI), carbimazole, high dosages of Vitamin A, Diethylstilbestrol, and other teratogenic medicines are also well-known. Diethylstilbestrol has been found to be both carcinogenic and teratogenic.
All three types of agents, i.e. mutagens, carcinogens, and teratogens, have one thing in common: they are all very powerful and exhibit their impact at very low doses.
Effects of Mutagens
Changes in a cell’s genetic makeup are known as mutations. These mutations can be fatal or non-lethal, and they can also be inheritable or non-inheritable. Mutations, in any event, change the genetic makeup of a population.
Many mutations can result in a variety of illnesses. Certain mutational illnesses are passed down through the generations and are caused by mutations in the germ cells.
Sickle cell anaemia is a condition caused by a single missense mutation in the -globin gene at codon 6 in germ cells. The glutamic acid at position 6 in the normal protein is replaced by valine in this mutation. This alteration has a significant impact on haemoglobin, the oxygen-carrying protein. The oxygen-carrying capacity of mutant haemoglobin is greatly decreased, and erythrocytes become stiff, resulting in painful blood cell passage, capillary blockage, and tissue injury.
The faulty erythrocytes are resistant to malaria, so the mutation has been passed down through the African population. Retinoblastoma or retinal tumours in children, Tay-Sachs disease, phenylketonuria, colorblindness, and cystic fibrosis are some of the illnesses caused by mutation.
It’s crucial to remember, though, that mutations aren’t always fatal or detrimental. The vast majority of mutations are completely harmless. In truth, mutations are to blame for the changes in the gene pool that have led to the development of life on the planet throughout the centuries.
Populations with a wide range of mutations have been able to withstand natural selection and adapt to meet their needs. Populations that were unable to adapt or change in response to changes in their surroundings eventually died out.
Mutation is, in reality, the initial step toward evolution. One such evolutionary genetic step is the development of coat colour by insects and animals for concealment. Apolipoprotein A1-Milano, a mutant protein Apolipoprotein, was discovered in a tiny Italian town with an unusual advantageous mutation (or Apo A1M).
Normal apolipoprotein is the protein responsible for cholesterol transport. Apo A1M, a mutant form of Apolipoprotein, not only eliminates cholesterol but also dissolves plaques and possesses antioxidant properties. As a result, the Italian community with the altered Apo A1M gene is protected against heart disease.
Mutagen Citations
- Mutagen formation during commercial processing of foods. Environ Health Perspect . 1986 Aug;67:75-88.
- Mutagenicity and genotoxicity studies of aspartame. Regul Toxicol Pharmacol . 2019 Apr;103:345-351.
- Is fluoride a mutagen? Sci Total Environ . 1988 Jan;68:79-96.
- Mutagen sensitivity assays in population studies. Mutat Res . 2003 Nov;544(2-3):273-7.
- Mutagen sensitivity: a genetic predisposition factor for cancer. Cancer Res . 2007 Apr 15;67(8):3493-5.
Share
Similar Post:
-
Myocardium: Definition, Function, and Examples
Continue ReadingMyocardium Definition
The myocardium is the muscular layer of the heart. It consists of cardiac muscle cells (cardiac myocytes [also known as cardiac rhabdomyocytes] or cardiomyocytes) arranged in overlapping spiral patterns.
What is Myocardium?
It is the heart’s muscular middle layer, wedged between the epicardium and the endocardium. It is sometimes referred to as the cardiac mass. Cardiac muscle cells (also known as heart muscle cells, cardiomyocytes, cardiac myocytes, or cardiac rahbdomyocytes) and fibroblasts make up the myocardium.
The heart is the human body’s primary circulatory organ, which pumps blood throughout the body. The heart is housed in the “pericardium,” a thin fibroelastic, double-layered, fluid-filled sac. The outer fibrous or parietal pericardium and the interior serous or visceral pericardium are the two layers of the pericardium.
Myocardium Etymology
The middle layer of the heart’s wall; the muscular material of the heart lying in the middle, i.e. between the epicardium and the endocardium (biology term). Mûs + karda are two Ancient Greek words that mean “muscle” and “heart,” respectively.
Heart Structure
The heart’s walls are divided into three layers:
1. Epicardium: The epicardium is the heart’s outermost layer. Underneath the mesothelial cells lie connective and adipose tissues. The visceral layer of the serous pericardium is likewise made up of this layer. There are coronary arteries and veins, lymphatic vessels, and nerves beneath the epicardium.

2. Myocardium: This is the heart’s muscle layer, which is responsible for the heart’s pumping activity. It accounts for 95 percent of the mass of cardiomyocytes and is the thickest layer in the heart wall. The pressure present in each chamber determines the thickness of the myocardial layer. As a result, the issue arises as to which of the four chambers of the heart has the thickest myocardium. The atrium has a thin myocardial layer, whereas the ventricles, particularly the left ventricle, have the thickest myocardium.
In fact, the left ventricular myocardium in mature animals is three times thicker than the right ventricular myocardium. This is fascinating, and it raises the question of why the left ventricle is thicker than the right. Because the left ventricle pumps blood across the body and also against the higher pressure in contrast to the right ventricle, the left ventricle’s walls are thicker than the right ventricle’s.
3. Endocardium: This is the deepest layer of the heart that borders the inner wall as well as the heart valves. The endocardium is further split into two layers:
(a) an inner endothelial cell layer that borders the heart chamber.
(b) a subendocardial layer that continues the connective tissue of the myocardial connective tissue layer. This subendocardial layer houses the heart’s impulse-conduction mechanism.
The heart is a muscular organ in vertebrates that pumps blood to various areas of the body. It circulates blood by causing rhythmic contractions. The heart’s wall is made up of three layers: the epicardium (outermost), the myocardium (middle), and the endocardium (innermost) (innermost).
Cardiac Muscle Histology
It is critical to comprehend the meaning of cardiac muscles. In a nutshell, cardiac muscles are striated, involuntary muscles made up of cardiac muscle cells, or cardiomyocytes.

One of the three types of muscles identified in vertebrates are cardiac muscles (the other two being skeletal muscles and smooth muscles). Cardiac muscles have certain characteristics of both smooth and skeletal muscles. Cardiac muscles, like skeletal muscles, are striated and may cause powerful contractions as well as begin continual contractions. Cardiac muscles, on the other hand, have certain distinct features.

The cardiomyocytes are attached to the fibrous skeleton of the heart in a helical or overlapping spiral arrangement. This helical pattern produces a three-dimensional structural network that is complicated.
Cardiac muscle fibres are elongated cylindrical in form, with a length of 50–100 m and a width of 10–25 m. The muscle fibres are made up of discrete quadrangular cells with a clear oval nucleus in the centre. Muscle cells that are rectangular in shape are frequently branched and connected end to end to create a syncytium.
An intercalated disc is formed when two cardiac muscle cells come together to produce a unique junctional complex. Three key components are included in these intercalated discs:
1. Desmosomes connect the cytoskeleton’s intermediate filaments.
2. Gap junctions with low electrical resistance that allow excitation to spread.
3. Adhering junctions, also known as fascia adherens, are linkages between actin filaments that allow contraction to be transmitted.
An intercalated disc is a structural term that refers to the double membranes created by closely linked cells and desmosomes joined by gap junctions. This is necessary for the transmission of electrical impulses from one cell to the next.
Gap junctions facilitate the electrical connection of muscle cells, allowing the heart muscles to pulse in unison. Cardiac muscle cells can create powerful, continuous, and rhythmic contractions on their own.
The autonomic nervous system and hormones, on the other hand, can affect the contractility of heart muscle cells. Intercalated discs appear as faint lines perpendicular to the long axis of the heart muscle fibres under a microscope.
A brown colour pigment situated in a perinuclear location may generally be detected in an adult human. The buildup of lipids, phospholipids, and proteins following lipid peroxidation is the brown colour pigment “lipofuscin,” which is called a “wear and tear” pigment.
The cardiac myocyte’s functional unit for contraction is the sarcomere. The contractile fibres of the sarcomeres are encircled by transverse discs known as Z-bands within each myocyte. A number of sarcomeres are stacked end to end and circumferentially in each myocyte, producing a “cable effect” within the cell.
Myosin and actin are two essential proteins present in heart muscle cells. The heavy filaments are made up of myosin, whereas the thin filaments are made up of actin. These two proteins work together to produce the myofibrillar filament, which is responsible for contractile activity in cardiac muscle tissues.
Due to the high energy demand for regular contractions, cardiac muscle cells’ sarcoplasm (i.e., the cytoplasm of cardiac myocytes) is unusually abundant in mitochondria. The cardiac myocytes use oxidative phosphorylation to satisfy their energy needs. As a result, these muscles demand a constant supply of oxygen. Coronary arteries transport blood to the myocardium. Glycogen granules are also found in the myofibrils to support the increased energy demand.
The myocardial has the highest oxygen demand and is the most severely impacted by reduced blood flow. Ischemia, or a reduction in blood flow, can be dangerous for these muscles. Cardiovascular muscles are also resistant to exhaustion. Myogenic in nature, cardiac muscles generate their own action potential. However, some modified muscle cells are specialised to generate the stimulus for the heartbeat and convey the impulse to various parts of the myocardial system.
The Sinoatrial node, atrioventricular node, bundle of His, and Purkinje fibres are all specialised cells that make up the cardiac conduction system. Contractile cells make up 99 percent of the myocardium, whereas the myocardial conducting system makes up only 1% of the cells.
Conducting myocardial cells are specialised muscle cells that work similarly to neurons. The action potential is initiated and distributed throughout the heart by these conducting cells, and the contracting myocardial cells pick it up to pump the heart rhythmically and regularly.
Myocardium Function
It is critical to comprehend the function of the myocardial in order to comprehend the circulatory system. The cardiac muscles’ principal role is to promote heart contractions and relaxation.
The contraction of the cardiac muscles causes blood to be pumped from the ventricles to the rest of the body, while the relaxation of the cardiac muscles enables blood to enter the atrium. The heart’s pounding circulates blood throughout the body, ensuring that every cell and tissue receives oxygen and nutrients.
Cardiac muscles are primarily controlled by an independent neurological system that sends out timed nerve impulses to the heart cells, causing them to contract and relax in a regular manner.
Myocardium Physiology
The sarcoplasmic reticulum is stimulated by an action potential or nerve impulse, resulting in the release of calcium ions (Ca2+) into the cytoplasm. Troponin is induced to release tropomyosin by cytoplasmic Ca2+ ions. Tropomyosin that has been released changes its location, allowing myosin to bind to actin.
The stored ATP molecules are subsequently used by myosin to shorten each sarcomere. In the absence of the impulse, however, fast Ca2+ reabsorption into the sarcoplasmic reticulum occurs. Troponin re-anchors itself to tropomyosin in the absence of Ca2+, resulting in cardiac muscle cell relaxation. Twitch contractions occur in the heart muscle, with a protracted refractory time followed by short relaxation intervals. The heart has to relax in order to fill the atrium with blood for the next cycle to begin.
The force on the walls of the heart chamber is generated when all of the cardiac muscles act in unison. The cardiac muscle sheets are arranged in a planar pattern, with each muscle perpendicular to the others. When the heart contracts, it does so in numerous directions as a result of this. As a result of the contraction of the numerous layers of cardiac muscle fibre, the ventricle and atrium decrease from top to bottom and side to side. This causes the ventricles to pump and twist vigorously, pushing blood throughout the body.
It’s crucial to remember that cardiac muscle passes via aerobic metabolism, which mostly uses lipids and carbs.
Myocardial Dysfunction
Primary (genetic) and secondary (non-genetic) cardiac disorders are the two kinds of myocardial dysfunction (mostly acquired but may be precipitated for genetic reasons).
i. Cardiac Myopathy
Cardiomyopathies are heart illnesses caused by a faulty myocardium. Cardiomyopathy is one of the leading causes of illness and death worldwide. Cardiomyopathies are classified into five groups based on their clinical manifestations:
1. Dilated Cardiomyopathy: Ventricular dilation and congestive heart failure symptoms. Some of the primary contributing causes of this disease are arterial hypertension, myocarditis, alcohol misuse, or tachyarrhythmias.
2. Hypertrophic Cardiomyopathy: Hypertrophic cardiomyopathy is caused by ventricular hypertrophy, particularly in the left ventricle. This cardiomyopathy is thought to be passed on through the generations via the sarcomere.
3. Restrictive Cardiomyopathy: Scarring and stiffness of the ventricle walls, as well as impaired diastolic filling of the heart, are seen in this condition.
4. Arrhythmic Cardiomyopathy: Arrhythmic cardiomyopathy is caused by genetically faulty desmosomes. Non-ischemic cardiomyopathy is characterised by arrhythmias. The bulk of the instances recorded had an issue with the right ventricle, but there have been a few occurrences of left ventricles as well.
5. Unclassified Cardiomyopathy: Unclassified cardiomyopathy is a kind of cardiomyopathy that does not fit into any of the other categories.
ii. Myocardial Infarction (Heart Attack)
The heart tissues demand a large amount of oxygen and energy that is delivered continuously. The coronary arteries carry oxygen from the lungs to the heart. These arteries, on the other hand, are predisposed to the development of atheromas, which are aberrant deposits of fatty acids, cholesterol, and other cell detritus. If these atheromas are big, they impede or decrease blood and oxygen flow to the cardiac cells, resulting in a disease known as myocardial infarction or heart attack.

Myocardial ischemia is a condition in which the oxygen flow to the heart cells is reduced. The absence of oxygen causes heart tissue to die. However, the damaged region is healed as part of the human body’s natural physiological reaction. Though the mending causes fibrous tissue to grow at the location, this disrupts the usual propagation and conduction of excitatory impulses, resulting in aberrant cardiac contractions. These asynchronous contractions cause cardiac arrhythmias, or irregular heartbeats, such as ventricular fibrillation.
iii. Cardiomyopathy
Cardiomyopathy is a heart muscle illness that lasts a long time.
1. Ischemic Cardiomyopathy: Diffuse coronary artery disease can cause prolonged heart ischemia, resulting in dilated cardiomyopathy. This can happen after one or more silent myocardial infarction events.
2. Metabolic Cardiomyopathy: Diabetes mellitus causes high blood glucose levels, which leads to cardiac dysfunction and metabolic cardiomyopathy.
3. Peripartum Cardiomyopathy: Peripartum cardiomyopathy is defined as left ventricular systolic dysfunction occurring within one month after childbirth or five months after delivery.
4. Tachycardia-induced Cardiomyopathy: Tachycardia-induced cardiomyo-pathy is caused by a chronic and consistently high heart rate (> 110 beats per minute), as observed in prolonged ventricular tachycardia or atrial fibrillation. It can eventually lead to cardiac failure if it is not addressed.
If cardiomyopathy is not addressed, it might result in the following complications:
1. Heart Failure: Ineffective heart pumping leads to inadequate blood to satisfy your body’s demands, which can be fatal.
2. Blood Clots: Blood clots can form, and these clots can limit blood supply to organs such as the heart and brain, finally leading to stroke.
3. Valve Problems: Cardiomyopathy can cause the heart to expand, causing the heart valves to close incorrectly.
4. Cardiac Arrest and Rapid Death: Cardiomyopathy can cause cardiac arrhythmias, which can cause fainting or death.
iv. Myocarditis
Myocarditis is caused by inflammation of the myocardium. This might be due to a number of factors, including viral infection (infectious myocarditis), toxic chemicals, medication allergies, bacterial, fungal, or parasite infection, and an autoimmune disease. In myocarditis, both cardiomyocytes and cardiac vascular endothelial cells can be damaged and lost. The white blood cells infiltrate the heart muscle wall as a result of this.
Interstitial cardiac fibrosis, wall motion anomalies, arrhythmias, heart failure, myocardial infarctions, decreased ejection fraction, and sudden cardiac death can all occur as a result of this. Chest discomfort, dyspnea, and flu-like symptoms are common symptoms of myocarditis, although it can also be asymptomatic.
v. Heart Failure
Heart failure or congestive heart failure, is a frequent end-stage route and symptom of cardiac dysfunction that can occur for a number of pathophysiological reasons. Heart failure occurs when the heart is unable to pump enough blood throughout the body, causing congestion, decreased organ perfusion, and functional impairment. Heart failure can be classified as acute or chronic, right heart vs. left heart, or systolic vs. diastolic, depending on the cause, location, and duration.
Each of these disorders has its own clinical manifestations and features. Myocardial injury or infarction, persistent hypertension, valve dysfunction, arrhythmias, cardiomyopathies, and other causes and risk factors can all lead to heart failure. Shortness of breath, extreme fatigue, and leg edema are all frequent signs of coronary heart failure. The use of medicines such as ACE inhibitors and beta-blockers, among other things, is crucial in the treatment of heart failure.
vi. Perioperative Myocardial Injury
Perioperative myocardial damage is a myocardial complication that can develop after non-cardiac surgery and is often underappreciated. It’s important to emphasise that this condition is not the same as a myocardial infarction. Perioperative myocardial damage is more likely in patients under the age of 65 who have a history of atherosclerotic disease.
There is a significant rise in cardiac troponin T plasma concentration in this situation (hs-cTn). This disease is frequently diagnosed and exhibited without the presence of chest discomfort, dyspnea, or other classic heart damage signs. Because most of the typical symptoms are absent, this disease is frequently misdiagnosed.
It is critical to do hs-cTn screening perioperatively to rule out the occurrence of this syndrome. The risk of 30-day death after noncardiac surgery has been demonstrated to be substantially increased by perioperative myocardial damage.
Biological Importance of Myocardium
The heart is the body’s primary pumping organ. The pumping of blood throughout the body is caused by the contraction and relaxation of the heart. The cells and tissues of the body require a certain quantity of oxygen, which is provided by the circulating blood, for optimal functioning.
The myocardium, or cardiac muscles, are in charge of the heart’s rhythmic and continual contraction and relaxation, or pumping activity. Cardiac muscles are one of the first embryonic organs that form and operate throughout life. These muscles are part of a complicated system that includes coronary arteries, cardiac lymphatics, and autonomic nerves.
Cardiomyocytes make up the heart’s thickest layer. The myocardial is such a vital organ in the body that its malfunction can be fatal. Cardiovascular disease is the biggest cause of mortality in the globe. The myocardium is involved in a variety of cardiac disorders, resulting in contractile dysfunction, cell death, and ventricular pump failure.
Myocardium Citations
- Application of Stem Cell Technologies to Regenerate Injured Myocardium and Improve Cardiac Function. Cell Physiol Biochem . 2019;53(1):101-120.
- A Contemporary Look at Biomechanical Models of Myocardium. Annu Rev Biomed Eng . 2019 Jun 4;21:417-442.
- Therapeutic Cardiac Patches for Repairing the Myocardium. Adv Exp Med Biol . 2019;1144:1-24.
Share
Similar Post:
-
Polygenic Trait: Definition, Types, and Examples
Continue ReadingPolygenic Trait Definition
A polygenic trait is a characteristic, sometimes we call them phenotypes, that are affected by many, many different genes. A classic example of this would be height.
What is a Polygenic Trait?
A polygenic trait is one in which a number of non-allelic genes play a role. These sorts of genes are referred to as polygenes. They are a collection of genes that, when activated, express as a single unit. Each of these has an influence on the characteristics as a whole. Nonetheless, it is difficult to differentiate the influence of a single gene, especially when a polygenic trait comprises many genes.
The phenotypic character that results is frequently an intermediate of heritable characteristics, although traits at the extremes are less common than intermediates. As a result, the polygenic trait distribution may be represented as a bell-shaped pattern with continuous fluctuation.
The various trait outcomes can not be readily and straightforwardly divided into groups, such as white or black, because of the broad range of trait variances, but rather by a wide spectrum, such as light to dark.
Polygenic characteristics in humans include height, skin colour, hair colour, and eye colour. Polygenic diseases include type 2 diabetes, coronary heart disease, cancer, and arthritis. However, polygenes can be impacted by environmental variables, so these circumstances are not solely genetic.
Polygenic Trait Etymology
The phrase polygenic derives from the words poly, which means “many,” and genic, which means “of genes.”
Polygenic Trait vs Mendelian Inheritance
A polygenic trait is one that develops as a result of polygenic inheritance. A non-Mendelian inheritance is one that does not obey Mendelian rules. Gregor Mendel, an Austrian monk and botanist, proposed the Mendelian rules. From 1856 until 1863, his breeding efforts and research on garden pea plants went undetected.
They were only discovered in the early twentieth century. More than three decades later, separate investigations by Erich von Tschermark, Hugo de Vries, Carl Correns, and William Jasper Spillman confirmed Mendel’s results. Monogenic inheritance, in which only one pair of alleles or one gene is involved, is an example of Mendelian inheritance.
The phenotypic ratio of a test cross involving a single pair of alleles may be easily predicted using a Punnett square because it follows Mendelian rules such as the Law of Unit Characters, the Law of Segregation, and the Law of Independent Assortment.
However, additional research revealed that some modes of inheritance and the phenotypic ratio that resulted did not follow these principles. Polygenic inheritance is one of them. This is because this kind of inheritance is regulated by several genes (called polygenes), which can be found at various loci on different chromosomes and are not controlled by a single gene (or one pair of alleles).
These polygenes are more likely to be expressed together in order to create a certain phenotypic characteristic. A polygenic trait is a characteristic that is created by the expression of several genes. The Punnett square would indicate larger child differences from a test cross in polygenic inheritance, and hence would not be as clear as it is in monogenic inheritance.
Monogenic vs Polygenic Trait
As previously stated, a monogenic trait is one that is caused by the expression of a single gene or a pair of alleles. The seed colour of the garden pea would be an example of this in one of Mendel’s breeding experiments. The progeny of a cross between a true-breeding yellow-seeded pea and a true-breeding green-seeded pea generated exclusively yellow seeds.
The green seed characteristic resurfaced in the following generation (F generation), reappearing at a 3:1 ratio, meaning that for every three offspring generating yellow seeds, one produced green seeds. Mendel argued that the characteristic that was expressed (yellow) was dominant, while the one that was masked (green) was recessive, based on these results.
A monogenic characteristic, on the other hand, would be more forthright and may be divided into two groups, yellow or green in this example.
A polygenic trait is one that is caused by the expression of several genes. These genes seemed to have an additive influence on the offspring’s phenotypic.
Consider it a painting on an art canvas made by combining various colours and intensities. As a result, a polygenic character is a polygenic activity that occurs across time. When a single dominant allele is present, its expression is limited to a single unit rather than the entire characteristic.
As a result, a polygenic trait is quantitative as opposed to a qualitative monogenic trait. Because the polygenic characteristic is caused by many genes, the phenotypic ratio of the F generation would deviate from 3:1. Despite the fact that several genes influence a characteristic, their separate impacts are difficult to identify.
A polygenic trait’s sensitivity to environmental influences is another characteristic. Environmental factors, in addition to polygenes, may influence the polygenic characteristics.
Bell-shaped Distribution
When a monogene is expressed, it creates discontinuous variation; when a polygene is produced, it produces continuous variation. An intermediate type of polygenic characteristics is often exhibited. Only a few dominant and recessive types exist. As a result, frequencies and ratios are less effective in forecasting polygenic differences than they are in predicting monogenic differences.
Variations in polygenic inheritance are calculated and evaluated using variances, covariances, and averages. Polygenic character characteristics have a consistent continuous range of variation in their frequency (e.g. from the lowest to the highest, the thinnest to the thickest, the shortest to the longest, the brightest to the darkest, and so on.).
To put it another way, the range of variance is distributed in a bell-shaped pattern. As a result, polygenes open up a lot of options in terms of phenotypic characteristics.
Polygenic Trait Examples
A large number of human characteristics are polygenic. Height, skin colour, and eye colour are examples of polygenic characteristics expressed in humans. Polygenes have the benefit of generating a broader range of phenotypic and genotypic variability in the population.
Several genes control human height, resulting in a broad variety of heights in a community. A genome-wide association analysis discovered 697 genetic variations in 423 genomic loci that have a role in determining an adult human’s height. Non-genetic variables (such as diet) can impact the characteristics in addition to genetic predisposition.
As a result, predicting the height of the children based on the heights of the parents is difficult. Despite having a tall father, the kid may be short. Similarly, a tall child can be conceived by two short parents. In certain circumstances, the child’s height will fall somewhere between the parents’.
Skin colour, hair colour, and eye colour are all polygenic characteristics. These characteristics are determined by how much melanin is produced and deposited by the body. A person with many alleles related to melanin synthesis and deposition will have a dark complexion on their skin, hair, and eyes.
An individual with alleles for melanin synthesis and deposition in the skin but no alleles for melanin in the eyes, for example, will have a darker skin colour but a lighter eye colour. An individual with fewer alleles for melanin synthesis and deposition, on the other hand, will have a lighter complexion.
It’s important to distinguish between a polygenic trait and a codominant characteristic. Human blood type AB, for example, is not a polygenic characteristic. It’s more of a case of dominance. Blood type AB people have dominant alleles for A and B antigens on their red blood cells, which means they are expressed together.
Polygenic Trait Disorders
Polygenes produce a genetic condition known as polygenic disease. Type-2 diabetes is an example of a polygenic disorder that can progress to illness. Polygenes are thought to be the majority of the genes involved in this disease.
According to recent research, there are more than 36 genes involved in the increased risk of type 2 diabetes. The inheritance of the TCF7L2 allele, for example, can raise the risk of diabetes by 1.5 times.
Other medically significant polygenic diseases include hypertension, coronary heart disease, cancer, arthritis, and mental illness, in addition to type-2 diabetes.
These disorders, on the other hand, aren’t totally hereditary. There might also be environmental variables at play that enhance a person’s chances of contracting any of them. Nonetheless, because they are polygenes that operate cumulatively, inheriting many of the genes associated with them raises the risk.
Polygenic Trait Citations
- Polygenic risk scores: from research tools to clinical instruments. Genome Med . 2020 May 18;12(1):44.
- The personal and clinical utility of polygenic risk scores. Nat Rev Genet . 2018 Sep;19(9):581-590.
- An Expanded View of Complex Traits: From Polygenic to Omnigenic. Cell . 2017 Jun 15;169(7):1177-1186.
Share
Similar Post: