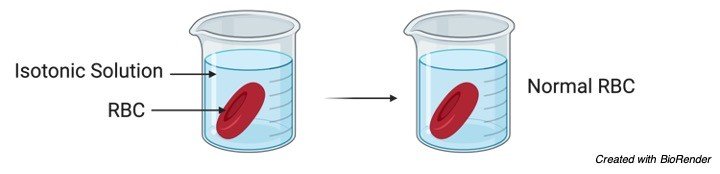
Isotonic Solution
The solution which has the same concentration in cell cytoplasm and in outer environment is referred to as isotonic solution.
This solution helps in movement of water into and outside of the cell, which is important for blood cells to perform their functions by delivering oxygen and nutrients to all parts of the body.
The effects of isotonic solution are generally referred to as isotonicity.
How Isotonic Solutions Work?
As isotonic solutions have same solute concentrations and they are separated by a semi permeable membrane, where water will flow in equal parts into and out of the solution.
But there will be no effect on the surfaces since water is moving across both sides.
Almost all organisms maintain their cells in an isotonic condition to support cellular functions.
Many organisms unlike plant cells which lack the cell wall mostly rely on the stability of external environment to maintain their shape and structure. Which also helps them to maintain their ph. and osmo -molarity of the cell.
Hence in isotonic solutions there will be no change or exchange in concentration of solutes but there will be movement of water into and out of the cells.
Physiology of Isotonic Solutions
Physiologically isotonic solutions contain the same osmotic pressure when compared with our body fluids such as blood and lachrymal fluids which are having their osmotic pressure similar to 0.9% of sodium chloride which in turn is said to be isotonic with plasma.
At the time of hospitalizations and while carrying out medical treatments for ph. adjustments in our body, the pharmaceutical solutions that are being used should be appropriate to the osmotic pressure of the body fluids so that there will be no swelling or discomfort occurs when they come in contact with our tissues.
One such pharmaceutical example is “Isotonic sodium chloride” which has same osmotic pressure as blood. It is commonly called as “Physiological saline solution” or “Normal saline solution”.
Effects of Isotonic Solutions
A. Plant and Animal Cells
Isotonic solutions are the one which has equal concentration in, in and out of the cell, which helps in diffusion of water in and out of the cell at equal rates, but there will be no diffusion of water across the plasma membrane which helps to retain the cells normal structure and function.
B. Blood Cells
We know that blood cells are embedded in a plasma. Here plasma is an isotonic solution when compared with blood cell cytoplasm, then the movement of water and nutrients in and out of the cells is feasible and this allows the cells to function normally.

Where the red blood cells are enriched with nutrients and oxygen and the waste products and carbon dioxide collected from the cells are passed out.
Imagine if we place the cells in a hypertonic environment without giving an equilibrium, due to higher concentration of the solutes in an outer environment the cells starts absorbing the salt contents and water and there is a risk of plasmolysis of those cells.
On considering other cases, if the cells are placed in a hypotonic environment where the concentration of solutes is lower in the outer environment, to equalize this condition; the cells state losing its nutrients, hence it is said that blood cells and many kind of human cells has its nature to sustain only in an isotonic environment.
To avoid these cases of plasmolysis or losing of nutrients the pharmaceutical fluids such as drip solutions are used only if it is in an isotonic condition.
Significance of Isotonicity in Human Physiology
It is important to exchange water and minerals constantly between the cells as it undergoes regular metabolic activities as we consume food and water regularly and excrete sweat, urine and faeces.
Without this mechanism to regulate isotonicity there is a high chance of accumulation of toxic substances and water in our cells.
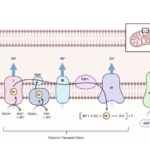
Mammalian systems are not only involved in regulating osmotic pressure and osmosis but also in maintaining the concentrations between extra cellular and intra cellular fluid and also the transfer of electrolytes and movement of water between the membranes.
It is very much important to maintain tonicity in blood plasma as it has direct effect on blood pressure.
Osmoconformers and Osmoregulators
In general, there are two different organisms which maintain the osmolarity of the environment and are known as Osmoconformers and the other which regulate the osmolarity of the body to be different from the environment and are known as osmoregulators.
The first group which is known as osmoconformers always exist in an isotonic solution as they have evolved to be the same concentration as of the environment.
This condition can be seen in many lower groups of organisms such as jelly fish, corals and sea slugs.
Whereas the osmoregulators do not exist in an isotonic solution, as this type of organisms needs its cell to remain functional all the time.
Hence both osmoregulators and osmoconformers have different benefits in the way of conducting their own life. But the isotonic solution is often created around the cells.
Hypotonic Solution : Definition and Examples
Citations
- Isotonic vs Hypotonic Intravenous Fluids for Hospitalized Children. JAMA . 2015 Aug 18;314(7):720-1.
- Question 3. Should isotonic infusion solutions routinely be used in hospitalised paediatric patients?. Arch Dis Child . 2011 Jun;96(6):608-10.
- Hypotonic vs isotonic saline solutions for intravenous fluid management of acute infections. Cochrane Database Syst Rev . 2004;2003(2):CD004169.