Author: Admin
-
Endoplasmic Reticulum: Definition, Types, Structure, and Location
Continue ReadingWhat is Endoplasmic Reticulum (ER)?
Inside the cytoplasm of most creature cells is a broad organization (reticulum) of film restricted channels, aggregately called the endoplasmic reticulum (or ER).
The endoplasmic reticulum is a name gotten from the way that in the light magnifying lens it’s anything but a “net in the cytoplasm.”
The endoplasmic reticulum is just present in the eukaryotic cells. In any case, the event of the endoplasmic reticulum differs from one cell to another.
For instance, the erythrocytes (RBC), egg and undeveloped cells need the endoplasmic reticulum.
Some bit of ER layers stays nonstop with the plasma film and the atomic envelope.
Features of Endoplasmic Reticulum (ER)
The membrane of the ER is 50 to 60 Aº thickness and liquid mosaic like the unit layer of the plasma film.
They are found to contain numerous sorts of compounds that are required for different significant manufactured exercises.

The main catalysts are the stearases, NADH-cytochrome C reductase, NADH diaphorase, glucose-6-phosphatase, and Mg++ enacted ATPase.
The layer of endoplasmic reticulum stays persistent with the films of the plasma layer, membrane, and Golgi apparatus.
The hole of the ER is all around created and goes about as a section for the secretory items.
Structure of Endoplasmic Reticulum
The ER may happen in the accompanying three structures: Lamellar structure or cisternae, Vesicular structure or vesicle and cylindrical structure or tubules.
I. The Cisternae
RER normally exists as cisternae that happen in those cells which have engineered jobs as the cells of the pancreas, notochord, and cerebrum.
The cisternae are for some time, smoothed, sac-like, unbranched tubules having a breadth of 40 to 50 μm.
They stay orchestrated parallelly in packs or stakes.
II. The Vesicles
The vesicles are oval; layer bound vacuolar structures having a distance across of 25 to 500 μm.
They frequently stay secluded in the cytoplasm and happen in many cells yet particularly bountiful in the SER.
III. The Tubules
The tubules are fanned designs shaping the reticular framework alongside the cisternae and vesicles.
They normally have a measurement from 50 to 190 μm and happen practically in every one of the cells.
Cylindrical type of ER is frequently found in SER and is dynamic in nature, i.e., it is related with film developments, splitting and combination between layers of cytocavity organization.
It might be unpleasant or smooth. The external surface of harsh ER has appended ribosomes, while smooth ER doesn’t have connected ribosomes.
The ER goes about as secretory, stockpiling, circulatory and sensory system for the cell. It is likewise the site of the biogenesis of cellular films.
Types of Endoplasmic Reticulum
I. Smooth Endoplasmic Reticulum
They are likewise called as the agranular endoplasmic reticulum. This kind of endoplasmic reticulum has smooth surfaces in light of the fact that the ribosomes are not joined to its layers.
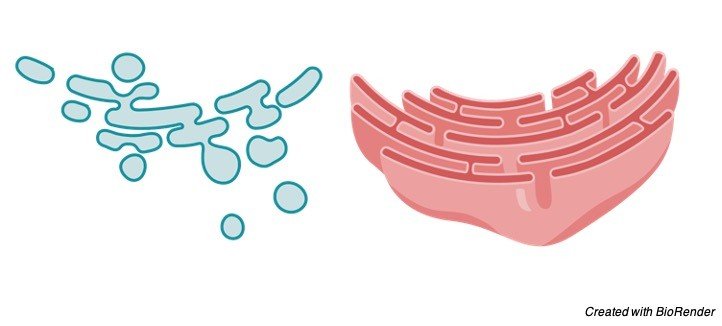
The smooth kind of endoplasmic reticulum happens generally in those cells, which are associated with the digestion of lipids (counting steroids) and glycogen.
Eg. fat cells, interstitial cells, glycogen putting away cells of the liver, conduction filaments of heart, spermatocytes, and leucocytes.
II. Rough Endoplasmic Reticulum
It has harsh walls on the grounds that the ribosomes stay connected to its layers. On their films, RER contains certain ribosome explicit, transmembrane glycoproteins, called ribophorins I and II, to which are joined the ribosomes while occupied with polypeptide union.
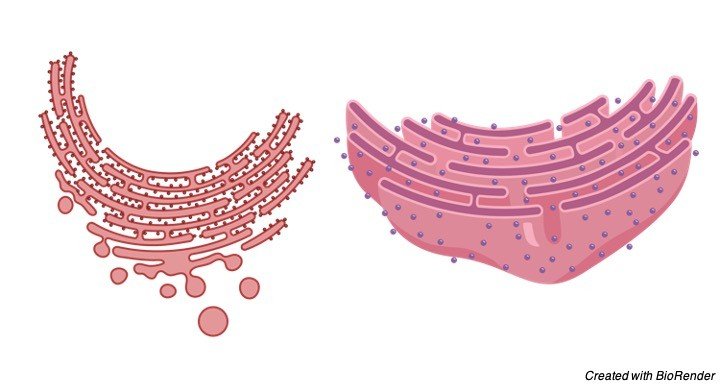
The unpleasant sort of ER is discovered plentifully in those cells which are dynamic in protein combinations like pancreatic cells, plasma cells, cup cells, and liver cells.
Function of Endoplasmic Reticulum
Elements of smooth ER incorporate lipid digestion (both catabolism and anabolism; they blend an assortment of phospholipids, cholesterol, and steroids).
Glycogenolysis (corruption of glycogen; glycogen being polymerized in the cytosol).
Medication detoxification (by the assistance of the cytochrome P-450). The endoplasmic reticulum gives a ultrastructural skeletal structure to the cell and gives mechanical help to the colloidal cytoplasmic network.
The trading of molecules by the cycle of assimilation, dispersion and dynamic vehicle happens through the layers of the endoplasmic reticulum.
The endoplasmic reticulum is the primary part of the endomembrane framework, additionally called the cytoplasmic vacuolar framework or cytocavity organization.
The endoplasmic layers contain numerous catalysts that perform different engineered and metabolic exercises.
Further, the endoplasmic reticulum gives an expanded surface to different enzymatic responses.
The endoplasmic reticulum goes about as an intracellular circulatory or shipping framework.
As a developing secretory polypeptide rises out of the ribosome, it goes through the RER film and gets aggregated in the lumen of RER.
Here, the polypeptide chains go through fitting, development, and sub-atomic collapsing to frame practical auxiliary or tertiary protein molecules.
RER squeezes off certain little protein-filled vesicles which at last get melded to cis Golgi.
The ER layers are found to lead intra-cellular driving forces. For instance, the sarcoplasmic reticulum communicates driving forces from the surface layer into the profound district of the muscle filaments.
The ER layers structure the new atomic envelope after each atomic division.
The SER contains a few key catalysts that catalyse the union of cholesterol which is likewise a forerunner substance for the biosynthesis of two sorts of mixtures—the steroid chemicals and bile acids.
RER additionally blend layer proteins and glycoproteins which are co-translationally embedded into the unpleasant ER layers.
In this manner, the endoplasmic reticulum is the site of the biogenesis of cellular layers.
Significance of Endoplasmic Reticulum
i. Transport of Materials: The ER works with transport of materials starting with one piece of the cell then onto the next accordingly framing the cell’s circulatory framework.
ii. Arrangement of Desmotubule: Tubular augmentation, called desmotubule, reaches out through plasmodesmata to make ER persistent in the two contiguous plant cells. Backing The ER goes about as an intracellular supporting system, the cytoskeleton that likewise keeps up with the type of the cell.
iii. Confinement of Organelles: It keeps the cell organelles appropriately positioned and dispersed according to one another.
iv. Surface for Synthesis: The ER offers broad surface for the blend of an assortment of materials.
v. Capacity of Materials: The ER gives space to impermanent capacity of manufactured items like proteins and glycogen. The ER helps in the trading of materials between the cytoplasm and the core.
vi. Area of Enzymes: An assortment of catalysts is situated in the ER to catalyse the biochemical responses.
Endoplasmic Reticulum Citations
- The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci . 2016 Jan;73(1):79-94.
- Endoplasmic reticulum stress signalling – from basic mechanisms to clinical applications. FEBS J . 2019 Jan;286(2):241-278.
- Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol . 2007 Jul;8(7):519-29.
- Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature . 2016 Jan 21;529(7586):326-35.
Share
Similar Post:
-
Bacterial Cell: Definition, Types, Structure, and Facts
Continue ReadingWhat are Bacterial Cells?
Bacteria are single-celled microorganisms. The cell structure is less complex than that of different creatures as there are no core or film-bound organelles.
A few bacteria have an additional circle of hereditary material called a plasmid.
The plasmid frequently contains qualities that give the bacterium some benefit over different bacteria.
For instance, it’s anything but a quality that makes the bacterium impervious to a specific anti-microbial.
History of Bacterial Cell
In the last part of the 1600s, Antoni van Leeuwenhoek turned into the first to consider bacteria under the magnifying instrument.
During the nineteenth century, the French researcher Louis Pasteur and the German doctor Robert Koch showed the part of bacteria as microbes (causing sickness).
The 20th century saw various advances in bacteriology, demonstrating their variety, antiquated genealogy, and general significance.
Most eminently, various researchers all throughout the planet made commitments to the field of microbial biology, showing that bacteria were vital for food networks and for the general soundness of the Earth’s environments.
The revelation that a few bacteria created compounds deadly to different bacteria prompted the improvement of anti-infection agents, which changed the field of medication.
Types of Bacterial Cell
Bacteria are ordered into five categories as per their fundamental shapes: circular (cocci), bar (bacilli), twisting (spirilla), comma (vibrios) or wine tool (spirochaetes).
They can exist as single cells, two by two, chains or bunches.

Bacterial Cell Residence
Bacteria are found in each territory on Earth: soil, rock, seas, and surprisingly cold snow. Some live in or on different organic entities including plants and creatures including people.
Bacterial cells are higher in number that human cells. A great deal of these bacterial cells is discovered coating the stomach-related framework.
A few bacteria live in the dirt or on dead plant matter where they assume a significant part in the cycling of supplements.
A few sorts cause food decay and harvest harm yet others are staggeringly helpful in the creation of aged food sources, for example, yogurt and soy sauce.
Somewhat a couple of bacteria are parasites or microorganisms that cause infection in creatures and plants.
Bacterial Cell Structure
i. Capsule
Some types of bacteria have a third defensive covering, comprised of polysaccharides (complex sugars). The most significant are to hold the bacterium back from drying out and to shield it from phagocytosis (overwhelming) by bigger microorganisms.
The capsule is a significant destructiveness factor in the significant sickness causing bacteria,
for example, Escherichia coli and Streptococcus pneumoniae.
Nonencapsulated freaks of these creatures are avirulent, for example they don’t cause infection.

ii. Cell Envelope
The cell envelope is comprised of a few layers: the inside cytoplasmic film, the cell divider, and – in certain types of bacteria – an external capsule.
iii. Cell Wall
Each bacterium is encased by an inflexible cell case made out of peptidoglycan, a protein-sugar (polysaccharide) and molecule.
It gives the cell its shape and encompasses the cytoplasmic layer, shielding it from the climate.
It likewise assists with securing limbs like the pili and flagella, which begin in the cytoplasm layer and jut through the divider to the outside.
The strength of the divider is liable for holding the cell back from blasting when there are huge contrasts in osmotic pressing factor between the cytoplasm and the climate.
When presented to a gram stain, gram-positive bacteria hold the purple shade of the stain.
In gram-negative bacteria, it is slender and discharges the colour promptly when washed with a liquor or CH3)2CO arrangement.
iv. Cytoplasm
The cytoplasm, or cellular material, of bacterial cells is the place where the capacities for cell development, digestion, and replication occurs.
It is a gel-like lattice made out of water, catalysts, supplements, squanders, and gases and contains cell constructions like ribosomes, a chromosome, and plasmids.
The envelope covers the most of its parts. Bacteria don’t have a film encased core.
The chromosome, a solitary, nonstop strand of DNA, is restricted, yet not contained, in a locale of the cell called the nucleoid.
The wide range of various cellular segments are dissipated all through the cytoplasm.
v. Plasmid
Plasmids are few, extrachromosomal hereditary designs conveyed by numerous strains of bacteria.
Like the chromosome, plasmids are made of a roundabout piece of DNA.
In contrast to the chromosome, they are not associated with proliferation. Just the chromosome has the hereditary features for starting and completing cell division, the essential method for generation in bacteria.
Plasmids imitate autonomously of the chromosome and, while not fundamental for endurance, seem to give bacteria a particular benefit.
Plasmids are given to different bacteria through two methods.
For most plasmid types, duplicates in the cytoplasm are given to little girl cells during binary fission.
Different sorts of plasmids, in any case, structure a tubelike design at the surface considered a pilus that passes duplicates of the plasmid to different bacteria during formation, an interaction by which bacteria trade hereditary data.
Plasmids have been demonstrated to be instrumental in the transmission of extraordinary properties, like anti-microbial medication opposition, protection from weighty metals, and harmfulness factors fundamental for disease of creature or plant has.
The capacity to embed explicit qualities into plasmids have made them amazingly valuable apparatuses in the fields of atomic science and hereditary qualities, explicitly in the space of hereditary designing.
vi. Cytoplasmic Membrane
A layer of phospholipids and proteins, called the cytoplasmic film, encases the inside of the bacterium, directing the progression of materials all through the cell.
This is a primary quality bacteria share with any remaining living cells; a hindrance that permits them to specifically interface with their current circumstance.
Films are profoundly coordinated and awry having different sides, each side with an alternate surface and various capacities.
Films are likewise unique, continually adjusting to various conditions.
vii. Flagella
Flagella (solitary, flagellum) are hairlike constructions that give a method for movement to those bacteria that have them.
They can be found at one or around its surface.
The flagella beat in a propeller-like movement to help the bacterium push toward supplements; away from poisonous synthetic compounds; or, on account of the photosynthetic cyanobacteria; around the light.
viii. Nucleoid
The nucleoid is an area of cytoplasm where the chromosomal DNA is found.
It’s anything but a film bound core, however essentially a space of the cytoplasm where the strands of DNA are found.
Most bacteria have a solitary, round chromosome that is liable for replication, albeit a couple of animal varieties do have at least two.
More modest round assistant DNA strands, called plasmids, are additionally found in the cytoplasm.
ix. Pili
Many types of bacteria have pili (solitary, pilus), little hairlike projections arising out of the external cell surface.
These outgrowths help the bacteria in joining to different cells and surfaces, like teeth, digestive organs, and rocks.
Without pili, numerous illness causing bacteria lose their capacity to taint since they’re not able to append to have tissue.
Specific pili are utilized for formation, during which two bacteria trade pieces of plasmid DNA.
x. Ribosomes
Ribosomes are factories and they decipher the hereditary code from the nucleic acid to that of amino acids—the structure squares of proteins.
Proteins helps in functioning of every one of the elements of cells and living life forms.
Bacterial ribosomes are like those of eukaryotes and are never bound to different organelles as they now and then are (bound to the endoplasmic reticulum) in eukaryotes, however, are unsupported constructions disseminated all through the cytoplasm.
There are adequate contrasts between bacterial ribosomes and eukaryotic ribosomes that a few anti-microbials will restrain the working of bacterial ribosomes, however not a eukaryote’s, along these lines killing bacteria yet not the eukaryotic life forms they are contaminating.
Bacterial Cell Citations
- Sculpting the bacterial cell. Curr Biol . 2009 Sep 15;19(17):R812-22.
- In situ probing the interior of single bacterial cells at nanometer scale. Nanotechnology . 2014 Oct 17;25(41):415101.
- Cytoprotective effect of proanthocyanidin-rich cranberry fraction against bacterial cell wall-mediated toxicity in macrophages and epithelial cells. Phytother Res . 2009 Oct;23(10):1449-52.
- The role of cytoskeletal elements in shaping bacterial cells. J Microbiol Biotechnol . 2015 Mar;25(3):307-16.
- The new bacterial cell biology: moving parts and subcellular architecture. Cell . 2005 Mar 11;120(5):577-86.
- Sporulation, bacterial cell envelopes and the origin of life. Nat Rev Microbiol . 2016 Aug;14(8):535-542.
Share
Similar Post:
-

Cell Nucleus: Definition, Function, Structure, & Facts
Continue ReadingWhat is Nucleus?
Nucleus (L. nucleus) is a particular two fold membrane attached protoplasmic body which contains all the hereditary data for controlling cell digestion and transmission to the successors.
A nucleus in the non-isolating or metabolic stage is called interphase nucleus. Like other cell structures, living perfect nucleus doesn’t show a lot of inward separation.
For an authentic investigation of the nucleus, the cells should be appropriately killed, fixed, and stained.
The nucleus is the biggest cell organelle.
However first saw by Leeuwenhoek in red blood corpuscles of fish, the nucleus was first concentrated in orchid root cells by Robert Brown in 1831.
Features of Nucleus
A nucleus is available in all living eukaryotic cells with the exception of develop strainer cells of vascular plants and red blood corpuscles of well evolved creatures.
Indeed, even here a nucleus is available during the beginning phases of their turn of events.
The presence of genetic data in the nucleus was demonstrated by crafted by Joachim Hammerling (1953) on single celled alga Acetabularia.
Total Count of Nucleus
Generally, cells are uninucleate, that is, they have a solitary nucleus.
The protistan Paramecium caudatum has two cores (bi-nucleate), macronucleus for controlling metabolic exercises of the creature and micronucleus possessing genetic data.
Multinucleate or polynucleate condition is found in certain cells of bone marrow, striated muscles, latex vessels, several parasites and green growth.
Multinucleate creature or protistan cells are called syncytial cells (e.g., epidermis of Ascaris) while in plants and growths they are called coenocytic cells (e.g., Rhizopus, Vaucheria).
Acellular sludge molds have a multinucleate protoplasmic body called Plasmodium.
Location of the Nucleus
Nucleus is typically found in the region of most extreme metabolic action in the cytoplasm.
Normally it is arranged in the mathematical focus of the cell.
In plant cells it is pushed to fringe position on one side because of the development of a huge focal vacuole.
It is peripheral in fat-putting away cells or adipocytes, and basal in glandular cells.
It is suspended in the focal vacuole by cytoplasmic strands in Spirogyra.
Composition of Nucleus
They have round border, and they seem oval or circular in plant cells having enormous focal vacuoles.
Plate like nucleus happen in the cells of squamous epithelium, lobed in white blood corpuscles and unpredictably stretched in silk turning cells of bugs.
DNA-9-12%. RNA-5%. Lipids-3%. Fundamental Proteins-15%. Corrosive proteins, impartial proteins and compounds 65%.
Hints of minerals like Calcium, Magnesium, Potassium and Sodium (Phosphorus is a constituent of DNA, RNA and corrosive proteins).
Structure of Nucleus
A regular interphase core is 5-25 pm in breadth. It is separated into five sections—atomic envelope, nucleoplasm, grid, chromatin and nucleolus.
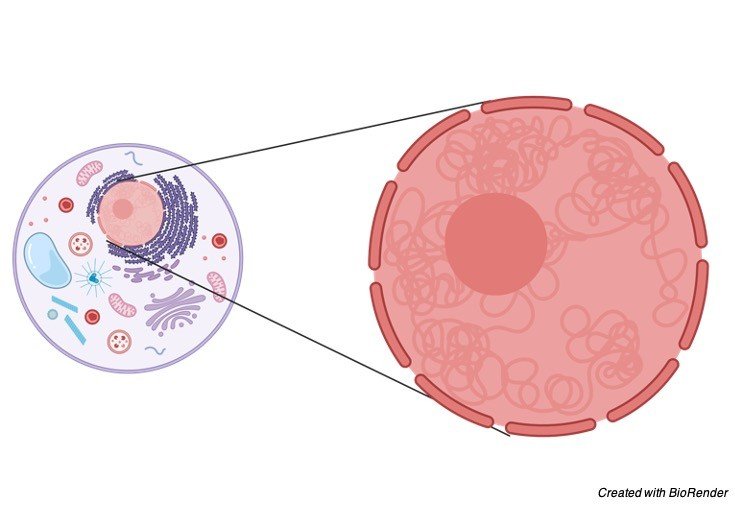
i. Envelope
It limits the core outwardly. The envelope isolates the core from the cytoplasm.
It is comprised of two lipoprotein and trilaminar films, every one of which is 60-90A thick.
The internal layer is smooth.
The external film might be smooth or its cytoplasmic surface may bear ribosomes like the unpleasant endoplasmic reticulum.
The two films of the atomic envelope are isolated by an electron straightforward perinuclear space.
The space is 100—500 An in width.
The external film is regularly associated with endoplasmic reticulum. It contains an enormous number of pores or holes.
At times 10% of the envelope is involved by pores.
The two films of the envelope become ceaseless in the locale of pores.
Pores have complex construction.
They may have stomach, septum, attachment of electron thick material or nucleoplasmin, blebs or annuli.
Annuli are round structures around the pores.
The pores and their annuli structure a pore complex called annulated pore.
An annulated pore may have 9 chambers, one focal and eight fringe.
All things being equal, there might be an organization of granules and fibers.
The pores control the entry of substances to within or outside of the core, e.g., RNAs, ribosomes, proteins.
ii. Nucleoplasm (Nuclear Sap, Karyolymph)
It is a straightforward, semifluid and colloidal substance which fills the core.
It contains nucleosides and various compounds (e.g., DNA polymerase, RNA polymerase, nucleoside phosphorylase) which are needed for the blend and working of DNA, RNA, nucleoproteins, and so on.
A portion of the proteins present in nucleoplasm are fundamental for shaft arrangement.
iii. Matrix
It is an organization of fine fibrils of corrosive proteins that capacity as platform for chromatin.
On the fringe, beneath the atomic envelope, atomic framework shapes a thick stringy layer called atomic lamina.
Terminal finishes of chromatin filaments or telomeres are installed in atomic or stringy lamina.
Atomic framework comprises of two kinds of intermediate fibers, lamin An and lamin B.
Framework and lamina structure:
(I) Scaffold for chromatin,
(ii) Attachment locales to telomeric parts,
(iii) Mechanical solidarity to atomic envelope, and
(iv) Components of atomic pore complex.
iv. Chromatin
It is innate DNA-protein fibrillar complex which is named so because of its capacity to get stained with certain essential colours.
Chromatin happens as fine covering and looped filaments which seem to create an organization called chromatin reticulum.
Chromatin filaments are conveyed all through the nucleoplasm.
They are separated into two districts—euchromatin and heterochromatin.
Euchromatin is tight (10-30nm thick) delicately stained and diffused sinewy part which shapes the majority of chromatin.
Heterochromatin is more extensive (100 nm thick), hazily stained and consolidated granular part which is joined to a great extent on the euchromatin.
Contingent on the size of granules shaped by heterochromatin they are called chromocentres, karyosomes or bogus nucleoli.
v. Nucleolus (plural-nucleoli)
It was first found by Fontana in 1781, depicted by Wagner in 1840 and furnished with its current name by Bowman in 1840.
Nucleolus is a stripped, round or marginally sporadic construction which is connected to the chromatin at a particular area called nucleolar coordinator locale (NOR).
Regularly 1-4 nucleoli are found in a nucleus.
Up to 1600 nucleoli are accounted for in the oocytes of Xenopus.
A covering layer is missing around nucleolus.
Calcium is by all accounts fundamental for keeping up with its arrangement.
Nucleolus has four segments—undefined framework, granular part, fibrillar segment and chromatin.
Function of Nucleus
It is a fundamental and vital piece of the eukaryote cell.
It stores hereditary data in its DNA particles which can be given to offspring girl cells.
It additionally controls cell functioning.
i. Chromatin
It contains genetic material called chromatin. Chromatin is DNA-protein complex. It is made of various fine filaments that gather to shape chromosomes. Number of chromosomes is fixed for an animal types and possess genes/qualities.
ii. Hereditary Information
Chromatin some portion of nucleus has all the hereditary data that is needed for development and improvement of the organic entity, its proliferation, digestion and conduct.
iii. Cell Activities
It controls cell digestion and different exercises through the development of RNAs (mRNA, rRNA, tRNA) which control union of specific kind of proteins.
iv. Ribosomes
Ribosomes are shaped in nucleolus part of the nucleus.
v. Varieties
All varieties are brought about by changes in hereditary material present in the nucleus.
vi. Cell Growth and Maintenance
With the assistance of RNAs, nucleus coordinates the blend of some underlying proteins and synthetic substances needed for cell development and support.
vii. Cell Differentiation
It coordinates cell separation by permitting certain specific arrangements of qualities to work.
vii. Cell Replication
Replication of nucleus is fundamental for cell replication.
Citations:
- The protozoan nucleus. Mol Biochem Parasitol . Sep-Oct 2016;209(1-2):76-87.
- The cell nucleus. A study in Burgundy. Nucleus . 2019 Dec;10(1):213-217.
- Chromatin’s physical properties shape the nucleus and its functions. Curr Opin Cell Biol . 2019 Jun;58:76-84.
- The nucleus: keeping it together by keeping it apart. Curr Opin Cell Biol . 2017 Feb;44:44-50.
Share
Similar Post:
-

TCA Cycle: Definition, Function, Mechanism, and Location
Continue ReadingWhat is TCA Cycle?
Tricarboxylic acid cycle, (TCA cycle), also called Krebs cycle and citrus acid cycle, the second stage of cellular respiration, the three-stage measure by which living cells break down organic fuel atoms within the sight of oxygen to harvest the energy they need to develop and isolate.
This metabolic interaction happens in many plants, animals, parasites, and many bacteria.
In all organisms aside from bacteria the TCA cycle is carried out in the matrix of intracellular designs called mitochondria.
History of TCA Cycle
The German British organic chemist Sir Hans Adolf Krebs proposed this cycle, which he called the citrus extract cycle, in 1937. For his work he got the 1953 Nobel Prize in Physiology or Medicine.
Although Krebs elucidated the majority of the reactions in this pathway, there were a few gaps in his plan.
The revelation of coenzyme A in 1945 by Fritz Lipmann and Nathan Kaplan allowed researchers to work out the pattern of reactions as it is known today.
Features of TCA Cycle
The TCA cycle plays a central job in the breakdown, or catabolism, of organic fuel particles—i.e., glucose and some different sugars, fatty acids, and some amino acids.
Before these rather large particles can enter the TCA cycle they should be degraded into a two-carbon compound called acetyl coenzyme A (acetyl CoA).
When taken care of into the TCA cycle, acetyl CoA is changed over into carbon dioxide and energy.
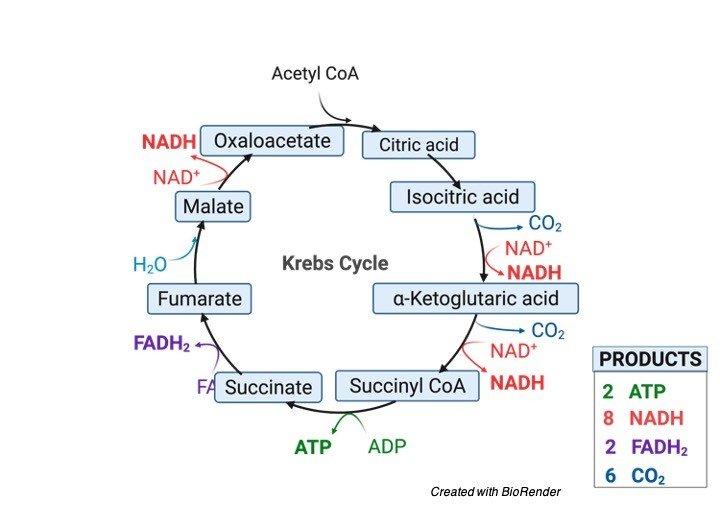
The TCA cycle comprises of eight stages catalyzed by eight distinct proteins. The cycle is initiated;
(1) when acetyl CoA reacts with the compound oxaloacetate to shape citrate and to release coenzyme A (CoA-SH). Then, at that point, in a progression of reactions;
(2) citrate is rearranged to frame isocitrate;
(3) isocitrate loses an atom of carbon dioxide and then, at that point goes through oxidation to shape alpha-ketoglutarate;
(4) alpha-ketoglutarate loses a particle of carbon dioxide and is oxidized to shape succinyl CoA;
(5) succinyl CoA is enzymatically changed over to succinate;
(6) succinate is oxidized to fumarate;
(7) fumarate is hydrated to create malate; and, to end the cycle,
(8) malate is oxidized to oxaloacetate.
Each total turn of the cycle brings about the regeneration of oxaloacetate and the formation of two particles of carbon dioxide.
Why TCA Cycle?
Energy is created in various strides in this pattern of reactions. In sync 5, one particle of adenosine triphosphate (ATP), the atom that powers most cellular capacities, is created.
A large portion of the energy obtained from the TCA cycle, in any case, is captured by the mixtures nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD) and changed over later to ATP.
Energy transfers happen through the relay of electrons starting with one substance then onto the next, an interaction carried out through the chemical reactions known as oxidation and decrease, or redox reactions. (Oxidation includes the deficiency of electrons from a substance and decrease the addition of electrons.)
For each turn of the TCA cycle, three particles of NAD+ are diminished to NADH and one atom of FAD is diminished to FADH2.
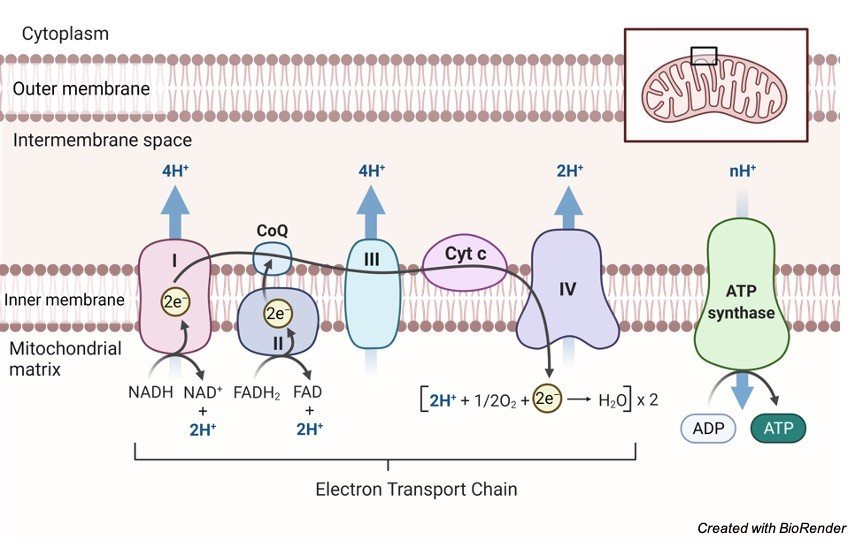
These atoms then, at that point transfer their energy to the electron transport chain, a pathway that is part of the third stage of cellular respiration.
The electron transport chain thusly releases energy so it tends to be changed over to ATP through the interaction of oxidative phosphorylation.
The TCA Cycle Net Equation
acetyl CoA + 3 NAD + FAD + ADP + HPO4-2 — > 2 CO2 + CoA + 3 NADH+ + FADH+ + ATP
Stage of TCA Cycle
Reaction 1: Formation of Citrate
The principal reaction of the cycle is that the condensation of acetyl-CoA with oxaloacetate to generate citrate, catalysed by citrate synthase.
Whenever oxaloacetate is gotten together with acetyl-CoA, a water particle attacks the acetyl leading to the release of coenzyme A from the complex.
Reaction 2: Formation of Isocitrate
The citrate is rearranged to frame an isomeric structure, isocitrate by a catalyst acontinase. In this reaction, a water atom is taken out from the citric acid and then, at that point set back on in another location. The overall impact of this change is that the – OH bunch is moved from the 3′ to the 4′ situation on the atom. This transformation yields the particle isocitrate.
Reaction 3: Oxidation of Isocitrate to α-Ketoglutarate
In this progression, isocitrate dehydrogenase catalyzes oxidative decarboxylation of isocitrate to frame α-ketoglutarate and NADH forms from NAD. The chemical isocitrate dehydrogenase catalyzes the oxidation of the – OH bunch at the 4′ situation of isocitrate to yield an intermediate which then, at that point has a carbon dioxide atom eliminated from it to yield alpha-ketoglutarate.
Reaction 4: Oxidation of α-Ketoglutarate to Succinyl-CoA
Alpha-ketoglutarate is oxidized, carbon dioxide is eliminated, and coenzyme An is added to shape the 4-carbon compound succinyl-CoA. During this oxidation, NAD+ is decreased to NADH + H+. The compound that catalyzes this reaction is alpha-ketoglutarate dehydrogenase.
Reaction 5: Oxidation of Succinate to Fumarate
Succinate is oxidized to fumarate. During this oxidation, FAD is decreased to FADH2. The compound succinate dehydrogenase catalyzes the removal of two hydrogens from succinate.
Reaction 6: Hydration of Fumarate to Malate
It is speed up by fumarase (fumarate hydratase). Fumarase proceeds with the rearrangement cycle by adding Hydrogen and Oxygen back into the substrate that had been recently taken out.
Reaction 7: Oxidation of Malate to Oxaloacetate
Malate is oxidized to create oxaloacetate, the starting compound of the citric acid cycle by malate dehydrogenase. During this oxidation, NAD+ is decreased to NADH + H+.
Total ATP Generation in TCA Cycle
Total ATP = 12 ATP
3 NAD+ = 9 ATP
1 FAD = 2 ATP
1 ATP = 1 ATP
Investigating the entire interaction, the Krebs cycle primarily transforms the acetyl gathering and water, into carbon dioxide and empowered types of different reactants.
Significance of TCA Cycle
Intermediate mixtures framed during TCA cycle are utilized for the blend of biomolecules like amino acids, nucleotides, chlorophyll, cytochromes and fats and so forth.
Intermediate like succinyl CoA takes part in the formation of chlorophyll.
Amino Acids are shaped from α-Ketoglutaric acid, pyruvic acids and oxaloacetic acid.
TCA cycle (citric Acid cycle) releases a lot of energy (ATP) needed for various metabolic activities of cell. By this cycle, carbon skeleton are got, which are utilized in interaction of development and for maintaining the cells.
TCA Cycle Citations
- Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun . 2020 Jan 3;11(1):102.
- The TCA cycle as a bridge between oncometabolism and DNA transactions in cancer. Semin Cancer Biol . 2017 Dec;47:50-56.
- Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys . 2014 Apr;68(3):475-8.
- The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell . 2018 Feb;9(2):216-237.
- Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Curr Opin Plant Biol . 2004 Jun;7(3):254-61.
Share
Similar Post:
-

Genotype vs Phenotype: Definition, Function, and Examples
Continue ReadingGenotype vs Phenotype
Any organic entity is a result of the two its genetic cosmetics and the climate. To comprehend this exhaustively, we should initially see the value in some essential genetic jargon and ideas.
Here, we give definitions to the terms genotype and phenotype, examine their relationship, and investigate why and how we may decide to contemplate them.
what is Genotype?
In science, a gene is a part of DNA that encodes a quality. The exact game plan of nucleotides (each made out of a phosphate gathering, sugar and a base) in a gene can vary between duplicates of a similar gene.
Along these lines, a gene can exist in various structures across creatures. These various structures are known as alleles.
The specific fixed situation on the chromosome that contains a specific gene is known as a locus. A diploid creature either acquires two duplicates of similar allele or one duplicate of two unique alleles from their folks.
On the off chance that an individual acquires two indistinguishable alleles, their genotype is supposed to be homozygous at that locus.
Notwithstanding, on the off chance that they have two distinct alleles, their genotype is classed as heterozygous for that locus.
Alleles of a similar gene are either autosomal predominant or latent. An autosomal predominant allele will consistently be specially communicated over a latent allele.
The ensuing blend of alleles that an individual has for a particular gene is their genotype.
Genotype Examples
A gene encodes eye tone. In this type, the allele is either brown, or blue, with one acquired from the mother, and the other acquired from the dad.
The earthy colored allele is prevailing (B), and the blue allele is passive (b). Assuming the youngster acquires two unique alleles (heterozygous) they will have earthy colored eyes. For the youngster to have blue eyes, they should be homozygous for the blue eye allele.
Different instances of genotype include:
• Hair tone
• Stature
What is Phenotype?
The amount of an organic entity’s noticeable attributes is their phenotype. A critical distinction among phenotype and genotype is that, while genotype is acquired from a living being’s folks, the phenotype isn’t.
While a phenotype is affected the genotype, genotype doesn’t approach phenotype.
The phenotype is impacted by the genotype and variables including: Epigenetic alterations, Natural and way of life factor.
Phenotype e.g.: Ecological variables that may impact the phenotype incorporate sustenance, temperature, dampness, and stress.
Flamingos are an exemplary illustration of what the climate means for the phenotype. While famous for being energetically pink, their normal tone is white – the pink tone is brought about by colours in the life forms in their eating routine. A subsequent model is a person’s skin tone.
Our genes control the sum and sort of melanin that we produce, nonetheless, openness to UV light in radiant environments causes the obscuring of existing melanin and supports expanded melanogenesis and consequently hazier skin.
Genotype vs Phenotype
Noticing the phenotype is straightforward – we investigate a living being’s outward highlights and attributes, and structure decisions about them.
Noticing the genotype, be that as it may, is somewhat more unpredictable.
Genotyping is the interaction by which contrasts in the genotype of an individual are examined utilizing natural measures.
The information got can then measure up against either a subsequent person’s arrangement, or a data set of groupings.
Already, genotyping would empower just incomplete successions to be acquired. Presently, because of major innovative advances as of late, cutting edge entire genome sequencing (WGS) permits whole groupings to be gotten.
A proficient interaction that is progressively reasonable, WGS includes utilizing high-throughput sequencing methods, for example, single-particle ongoing (SMRT) sequencing to distinguish the crude succession of nucleotides establishing a living being’s DNA.
WGS isn’t the best way to investigate an organic entity’s genome – an assortment of techniques are accessible.
Genotyping Techniques
• PCR
• DNA Microarray
• Allele-explicit Oligonucleotide (ASO) Probes
• DNA Hybridization
Understanding Genotype vs Phenotype
Understanding the connection between a genotype and phenotype can be very valuable in an assortment of examination regions.
An especially fascinating region is pharmacogenomics. Genetic varieties can happen in liver chemicals needed for drug digestion, like CYP450.
Hence, a person’s phenotype, for example their capacity to utilize a particular medication, may differ contingent upon which type of the chemical encoding gene they have.
For drug organizations and doctors, this information is key for deciding suggested drug measurements across populaces.
Utilizing genotyping and phenotyping strategies pair have all the earmarks of being superior to utilizing genotype tests alone.
In a similar clinical pharmacogenomics study, a multiplexing approach recognized more noteworthy contrasts in drug digestion limit than was anticipated by genotyping alone.
This has significant ramifications for customized medication and features the should be careful when solely depending on genotyping.
Utilizing creature models like mice, researchers can genetically adjust a life form, so it no longer communicates a particular gene – known as knockout mice.
By contrasting the phenotype of this creature with the wild sort of phenotype (for example the phenotype that exists when the gene has not been eliminated), we can contemplate the job of specific genes in conveying certain phenotypes.
The Mouse Genome Informatics (MGI) drive has aggregated a data set of thousands of phenotypes that can be made and contemplated and the genes that should be taken out to deliver every particular phenotype.
The genotype of the individual decides the phenotype. Notwithstanding, it’s anything but consistently conceivable to know the genotype by taking a look at the phenotype.
To sort out the genuine genotype, the family ancestry can be inspected or it very well may be reared in a test cross, and the posterity can show whether it had a secret hidden recessive allele.
Distinction Between Genotype vs Phenotype
Features Genotype Phenotype Definition The arrangement of genes in our DNA which are liable for a specific characteristic A living being’s perceptible attributes and characteristics Portrayed by Genotyping strategies like WGS Noticing a living being’s outward qualities Relies upon The gene arrangements a creature has Genotype, PLUS epigenetics and natural components Acquired? Yes No For instance Gene encoding the eye tone A person with earthy colored eyes Genotype vs Phenotype Citations
- Drilling for Insight: Forecasting Phenotype from Genotype. Trends Genet . 2018 Nov;34(11):821-822.
- Methods for genotyping single nucleotide polymorphisms. Annu Rev Genomics Hum Genet . 2001;2:235-58.
- Integrating large-scale genotype and phenotype data. OMICS . Winter 2006;10(4):545-54.
- Genotype Imputation in Genome-Wide Association Studies. Curr Protoc Hum Genet . 2019 Jun;102(1):e84.
Share
Similar Post:
-

Cytoskeleton: Definition, Importance, and Functions
Continue ReadingWhat are Cytoskeleton?
The presence of cytoskeleton in the design of the cellular material was proposed by Koltzoff in 1928. They are intertwining organization of protein fibers stretched out all through the cytoplasm and lattice of various proteins in cells.
As its name infers it assists with keeping up with cell shape and is significant in cell motility.
It is a unique three-dimensional construction that fills the cytoplasm. This construction goes about as both muscle and skeleton, for development, locomotion, and strength.
Features of Cytoskeleton
The interior movement of cell organelles, velocity, and muscle fiber withdrawal can’t happen without the cytoskeleton.
It is accepted that cytoskeleton is the trademark highlight of eukaryotic cells however late examination demonstrated that prokaryotic cells have proteins that structure a cytoskeleton.
Over the span of the human genome project more than 800 presumably cytoskeleton related qualities are found.
Based on three kinds of protein fibers, cytoskeletons are of three sorts like microtubules, halfway fibers and microfilaments.
Microfilaments
Microfilaments are fine, string-like protein strands, 5-7 nm in breadth, address the dynamic or motile piece of the cytoskeleton.
They seem to assume a significant part in cyclosis and amoeboid movement. They are made overwhelmingly out of a contractile protein called actin, which is the most plentiful cell protein.
These fibers are cross connected into organizations or groups. The semi adaptable microfilaments make cells versatile, to isolate in mitosis (cytokinesis) and are answerable for strong compression. The adaptable transitional filaments fortify the cell furthermore.
As a rule a shell of microfilaments upholds the plasma layer. Microfilaments are a polymer of actin protein subunits in addition to appended proteins like cross-linkers.
Most multi-cell creatures have a few actin iso-structures. People have six actin qualities; four encode alpha-actin, one beta-and one gamma-actin.
Alpha-actin is found in muscle cells where it assumes a significant part in getting the cell, though beta-actin is limited toward the front of moving cells and gamma-actin structures pressure strands.
Actin protein as a polymer without connected proteins is called filamentous actin (F-actin), while the globular actin monomers are called G-actin.
Actin Filaments
Actin fibers are 8 nm in distance across and comprise of two strands of the protein actin that are bound around one another.
They are particularly unmistakable in muscle cells, where they accommodate the withdrawal of muscle tissue, monomers of the protein actin polymerize to frame long, slight filaments.

A few elements of actin fibers structure a band just underneath the plasma layer that gives mechanical solidarity to the phone, joins trans-film proteins (e.g., cell surface receptors) to cytoplasmic proteins, secures the centrosomes at inverse posts of the cells during mitosis, and squeezes partitioning creature cells during cytokinesis.
It produces cytoplasmic gushing in certain cells, velocity in cells, for example, white platelets and the one-celled critter, and connect with myosin (“thick”) fibers in skeletal muscle strands to give the power of solid constriction.
Microtubules
Microtubules are profoundly unique protein polymers that structure an essential piece of the cytoskeleton in every single eukaryotic cell.
Robertis and Franchi (1953) noticed the first time in the axoplasm of the myelinated nerve filaments, which they called neurotubules.
Microtubules were first depicted exhaustively by Ledbetter and Porter (1963).
Microtubules are transport lines inside the cells.
Microtubules are round and hollow cylinders, 20-25 nm in measurement, and made out of protein tubulin subunits.
These subunits are named alpha and beta. Each miniature tubule is composed of eleven sets of these tubulin subunits masterminded in a ring.
A significant part of microtubules is giving a pathway to intracellular developments of organelles and proteins.
This is finished by engine proteins (kinesins and dyneins) under the utilization of ATP.
The centrosome is situated in the cytoplasm appended to the outside of the core. It is duplicated during the S phase of the cell cycle. Not long before mitosis, the two centrosomes move separated until they are on inverse sides of the core.
As mitosis continues, microtubules develop out from every centrosome with their in addition to closes developing toward the metaphase plate. These clusters of microtubules are called axle strands.
Importance of Cytoskeleton: Cell Movement
Cell movement is achieved by cilia and flagella. Cilia are hair-like constructions that can beat in synchrony causing the movement of unicellular Paramecium.
The two cilia and flagella are built from microtubules, and both give either motion to the cells (e.g., sperm) or move liquid (e.g., ciliated epithelial cells that line our air entries and move a film of bodily fluid towards the throat).
Every cilium or flagellum is made of a round and hollow exhibit of 9 equally divided microtubules, each with a halfway microtubule connected to it. 2 single microtubules run up through the focal point of the pack, finishing the supposed “9+2” design.
The whole get together is sheathed in a layer that is just an expansion of the plasma membrane.
Some eukaryotic cells move about through microtubules connected to the outside of the plasma layer. These microtubules are called flagella and cilia.
Flagella and cilia both have a similar construction: a ring of nine tubulin trios orchestrated around two tubulin sub-units.
The distinction among flagella and cilia lies in their movement and numbers. Flagella are appended to the cell by a “wrench” Mike mechanical assembly that permits the flagella to turn. Cilia, then again, are not joined with a “wrench,” and beat to and fro to give movement.
Ciliated cells generally have many these projections that cover their surfaces.
For instance, the protist Paramecium moves through a solitary flagellum, while the protist Didinium is covered with various cilia.
In microtubules one alpha-and one beta-tubulin structure a hetero-dimer. Long chains of these hetero-dimers made out of proto-fibers, wherein consistently an alpha-tubulin is followed by a beta-tubulin.
Each microtubule has a (- ) and a (+) end. At the (+) or beta-tubulin end new heterodimers are added quicker and at lower tubulin fixations than at the (- ) or alpha-tubulin end.
The alpha-tubulin just as the beta-tubulin subunit ties a little guanosine tri-phosphate (GTP). Cells have protein engines that tight spot two particles, and utilizing ATP as energy, influence one atom to move in relationship to the next.
Two sorts of these protein engines are myosin and actin, and dynein or kinesin and microtubules. These groups of proteins all have a motor end, however, may have a few sorts of various atomic designs on the limiting end.
At the point when these proteins tie the atoms they are moved to various organelles. When connected to different microtubules, protein engines can cause movement if the finishes are fixed or broaden the lengths of the fiber groups if the closures are free.
Cytoskeleton Citations
- Manipulation of the Host Cell Cytoskeleton by Chlamydia. Curr Top Microbiol Immunol . 2018;412:59-80.
- The cytoskeleton. Natl Cancer Inst Monogr . 1982;60:31-46.
- The Role of Host Cytoskeleton in Flavivirus Infection. Virol Sin . 2019 Feb;34(1):30-41.
- The structural organization of Giardia intestinalis cytoskeleton. Adv Parasitol . 2020;107:1-23.
- The Evolving Complexity of the Podocyte Cytoskeleton. J Am Soc Nephrol . 2017 Nov;28(11):3166-3174.
- The Cytoskeleton as a Modulator of Aging and Neurodegeneration. Adv Exp Med Biol . 2019;1178:227-245.
- The cytoskeleton as a novel therapeutic target for old neurodegenerative disorders. Prog Neurobiol . 2016 Jun;141:61-82.
- Cytoskeleton-a crucial key in host cell for coronavirus infection. J Mol Cell Biol . 2020 Jul 1;12(12):968-979.
- Overview of the Cytoskeleton from an Evolutionary Perspective. Cold Spring Harb Perspect Biol . 2018 Jul 2;10(7):a030288.
- The cytoskeleton and cancer. Cancer Metastasis Rev . 2009 Jun;28(1-2):5-14.
- Mechanical Properties of the Cytoskeleton and Cells. Cold Spring Harb Perspect Biol . 2017 Nov 1;9(11):a022038.
- Cell mechanics and the cytoskeleton. Nature . 2010 Jan 28;463(7280):485-92.
Share
Similar Post:
-
Cell Proliferation: Definition, Importance, and Benefits
Continue ReadingWhat is Cell Proliferation?
Cell proliferation is defined as the increment in cell count resulting because of cell division. It is a complex, firmly controlled, clear cut cycle.
The mechanisms of normal cell proliferation, just as the pathologic outcomes happening when the system malfunctions, are basic to numerous spaces of medication, from embryogenesis, to repair of tissues, and finally to oncogenesis.
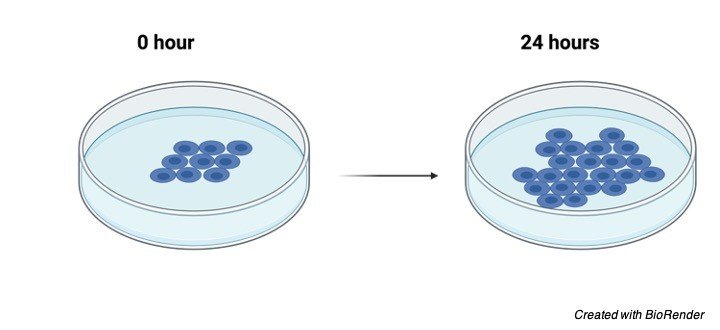
Cell Proliferation and Tissue Type
Firstly, few tissues contain cells that, previously terminally differentiated, are impotent to return the cell cycle. These tissues are known as nondividing tissues; a common representative is neural tissue.
Secondly, different tissues, like liver tissue, contain cells that typically live external the cell cycle, however which can be stimulated to multiply when needed. These tissues are called stable tissues.
Thirdly, the tissue type is named consistently dividing tissue, is persistently being supplanted because of continuous cell sloughing or cell demise. Skin and bone marrow are instances of this sort of cell.
Mechanism of Cell Proliferation
Cell division
Cells attempt the interaction of division by advancing through an exceptionally directed cycle called as the cell cycle, the end product being a duplication of the substance of the mother cell into two daughter cells.
The cell cycle was found by Prevost and Dumas (1824) while considering the cleavage of zygote of Frog.
It is a progression of stages a cell goes through, to divide and give rise to new cells.
Phases of Cell Division
Cell cycle or cell division alludes to the series of occasions that occur in a cell prompting its development and resulting division. These occasions incorporate duplication of its genome and synthesis of the cell organelles followed by division of the cytoplasm.
A commonplace eukaryotic cell cycle is separated into two primary phases:
i. Interphase
Otherwise called the resting period of the cell cycle; interphase is the time during which the cell gets ready for division by going through both cell development and DNA replication.
It involves around 95% time of the general cycle. The interphase is categorised into three phases:

• G1 phase (Gap 1): G1 phase is the period of the cell among mitosis and commencement of replication of the genetic material of the cell. During this phase, the cell is metabolically dynamic and keeps on developing without recreating its DNA.
• S phase (Synthesis): DNA replication happens during this phase. In the event that the underlying amount of DNA in the cell is signified as 2N, then, at that point after replication it gets 4N. The centriole additionally isolates into two centriole sets in the cells which contain centriole.
• G2 phase (Gap 2): During this phase, the RNA, proteins, different macromolecules needed for augmentation of cell organelles, spindle formation, and cell development are delivered as the cell plans to go into the mitotic stage.
A few cells like heart cells in the grown-up creatures don’t display division and some others just separation to supplant those cells which have been either harmed or lost because of cell death.
Such cells which don’t divide further achieve an idle G0 stage otherwise called quiescent phase after they leave the G1 stage.
These cells remain metabolically dynamic however don’t divide except if called upon to do as such.
The cycle of interphase comprises of;
Steps Phases Events 1 G0 Phase (Resting Phase) The cell neither divides nor sets itself up for the division. 2 G1 Phase (Gap 1) The cell is metabolically dynamic and develops consistently during this phase. 3 S phase(Synthesis) The DNA replication or synthesis happens during this phase. 4 G2 phase (Gap 2) Protein synthesis occurs in this phase. 5 Quiescent phase (G0) The cells that don’t undergo additional division leaves the G1 phase and enters a latent phase. This stage is known as the quiescent phase (G0) of the cell cycle. ii. M Pahse
This is the mitotic phase or the period of the equational division as the cell goes through a total rearrangement to bring forth a descendant that has similar number of chromosomes as the parent cell.
Different organelles are additionally separated similarly by the cycle of cytokinesis which is gone before by mitotic nuclear division.
The mitotic stage is separated into four overlapping stages:
1. Prophase
2. Metaphase
3. Anaphase
4. Telophase
Types of Cell Division
1. Mitosis: In this process, cells precisely replicates themselves. It is generally seen in multicellular organism possessing varying degree of cells.
For instance, cells of nerves, muscle, skin, hair, etc.
2. Meiosis: These are the cell division wherein sperm or egg cells are produced which contain half the number of chromosomes.
3. Binary Fission: Single celled micro-organisms duplicate themselves for precreation.
The phases of cell division and the transitions between the phases are co-ordinated by a multifaceted sequence of signalling mechanism.
At the point when the lost tissue is supplanted, the cells get back to the regular quiescent state, in this way restoring the cellular organ and tissue homeostatic systems.
Cell Proliferation and Stem Cells
The characterizing properties of a stem cells are
(1) The capacity to separate essentially unbounded all through the lifetime of the organic entity
(2) The capacity to divide symmetrically (prompting two terminally separated cells) or asymmetrically (bringing about one undifferentiated cell and one terminally separated cell).
Stem cells are required any place there is a repetitive need to supplant nondividing, terminally separated cells.
Some terminally differentiated cells, for example, develop mammalian red blood cells and the cells in the peripheral layer of the skin, do not have a cell nucleus and are accordingly incapable to isolate.
Some other contains cytoplasmic structure, (for example, the myofibrils of striated muscle cells) that obstruct cell duplication. Also, in some terminally separated cells, the science of separation may just be contradictory with cell division.
Stem cells that bring about just one type of differentiated cell are known as unipotent; these are equipped of differentiating along only single lineage. Also, few cell types are known as oligopotent. These stem cells can separate to differentiate into a couple types of cells.
A lymphoid stem cell is another sample of an oligopotent stem cell.
Cells that about numerous cell types are known as pluripotent or totipotent. However, there is different type of stem cell that is known as multipotent.
These cells are ancestor cells that have the genetic capability to differentiate into numerous, but restricted cell types.
Cell Proliferation and Cancer
Cells inside a tissue apply an inhibitory impact on one another’s development.
This limiting force is known as social control of cell division, and it is interceded by a set of genes called social control genes.
A cell that gets a DNA mutation that disrupts this social limitation will divide regardless of the necessities of the organism in general, and its offspring may become tumor cells.
Summary of Cell Proliferation
Maybe carefully incited suppression of pathways associated with cell passing and incitement of pathways associated with cell division that re-establish organ structure and function.
With the approach of gene therapy, explicit genes could, at some point, be conveyed straightforwardly to the damaged organ to incite articulation/concealment of the fitting variables required for recovery.
Cell Proliferation Citations
- Cell Proliferation and Cytotoxicity Assays. Curr Pharm Biotechnol . 2016;17(14):1213-1221.
- CXXC4 mediates glucose-induced β-cell proliferation. Acta Diabetol . 2020 Sep;57(9):1101-1109.
- Targeting mitosis exit: A brake for cancer cell proliferation. Biochim Biophys Acta Rev Cancer . 2019 Jan;1871(1):179-191.
- Mathematical models of tumor cell proliferation: A review of the literature. Expert Rev Anticancer Ther . 2018 Dec;18(12):1271-1286.
- Quantification of epithelial cell proliferation, cell dynamics, and cell kinetics in vivo. Wiley Interdiscip Rev Dev Biol . 2017 Jul;6(4).
- Tight Junctions in Cell Proliferation. Int J Mol Sci . 2019 Nov 27;20(23):5972.
- Regulation of cell proliferation by hypoxia-inducible factors. Am J Physiol Cell Physiol . 2015 Dec 15;309(12):C775-82.
Share
Similar Post:
-

ATP Synthase: Definition, Function, and Location
Continue ReadingWhat is ATP Synthase?
The ATP synthases are multiprotein buildings found in the energy-transducing films of microorganisms, chloroplasts and mitochondria.
They utilize a transmembrane protonmotive power, p, as a wellspring of energy to drive a mechanical rotating instrument that prompts the compound union of ATP from ADP what’s more, Pi.
Features of ATP Synthase
Their general design, association and unthinking standards are generally grounded, however different highlights are less surely knew.
For instance, ATP synthases from microbes, mitochondria and chloroplasts contrast in the systems of guideline of their movement, and the sub-atomic bases of these various instruments and their physiological jobs are as it were simply starting to arise.
Another essential component coming up short on a atomic depiction is the way revolution driven by p is produced, also, how pivot sends energy into the synergist locales of the protein to create the venturing activity during turn.
One amazing and deficiently clarified derivation dependent on the balance of c-rings in the rotor of the protein is that the measure of energy needed by the ATP synthase to make an ATP atom doesn’t have a general worth.
ATP synthases from multicellular living beings require the least energy, though the energy needed to make an ATP atom in unicellular living beings and chloroplasts is higher, and scope of qualities has been determined.
At last, the proof is developing for different jobs of ATP synthases in the internal films of mitochondria.
Here the chemicals structure supermolecular buildings, perhaps with explicit lipids and these buildings likely add to, or indeed, even decide the development of the cristae.
Structure of ATP Synthase
They comprise of two major utilitarian spaces, a layer extraneous F1 area furthermore, a film inherent Fo area consolidated by focal furthermore, fringe stalks.
The F1 area is the reactant part of the compound where ATP is shaped from ADP and Pi.
The Fo area contains an engine, which produces revolution utilizing the potential energy put away in p.
Schematic Representation of ATP Synthase
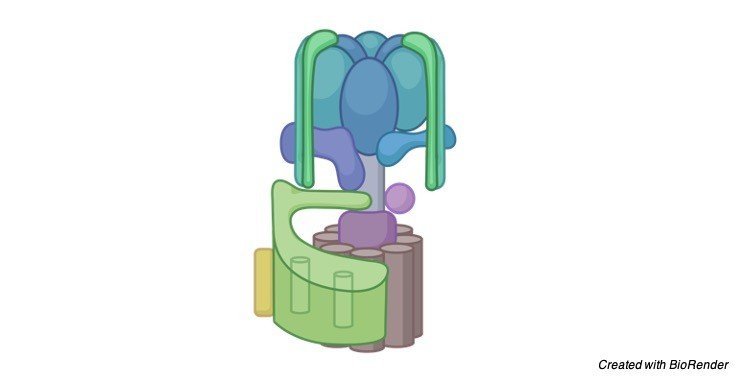
The rotational energy of the engine is communicated to the synergist area by the focal tail, which is joined straightforwardly to the turning engine.
The fringe tail interfaces the α3β3 area to subunit an in the Fo space, so together they structure the necessary stator of the compound.
The F1 area of the ATP synthase is a get together of five globular proteins, α, β, γ , δ and ε, with the stoichiometry α3β3γ 1δ1ε1.
The joined atomic mass of these subunits is around 350 kDa. In the α3β3γ 1 subassembly, which is normal to all ATP synthases, the three α-and the three β-subunits structure an around circular α3β3 structure around 100 A˚ (1 A˚ = 0.1 nm) in distance across, with the six subunits masterminded in shift around a prolonged α-helical design in the γ – subunit.
This piece of the γ – subunit is totally encompassed in the α3β3 area and involves its focal pivot.
In this way the α3β3 structure takes after an orange made of six fragments orchestrated around the focal substance tail of the organic product.
The rest of the γ – subunit projects roughly 30 A˚ past the α3β3 space and structures part of a ‘foot’ that joins the α3β3γ 1 complex solidly to the Fo area.
This connection is expanded by another protein, known for authentic reasons as the ε-subunit in microorganisms and chloroplasts, and as the δ-subunit in mitochondria.
Notwithstanding their various names, these ε-and δ-subunits are collapsed and presumably tie in a comparable way, and their capacities are presumably related likewise, in spite of the fact that they are not indistinguishable.
In mitochondria, the connection of the foot of the γ – subunit to the Fo space is increased by a subsequent subunit.
Referred to, confusingly, as the ε-subunit, this mitochondrial protein has no partner in bacterial and chloroplast catalysts.
The γ – subunit, what’s more, the related ε-subunits in the bacterial and chloroplast proteins, and the related δ-and ε-subunits in the mitochondrial protein, are bound immovably to one finish of a hydrophobic barrel shaped design made of a ring of csubunits in the Fo film space of the chemical.
This chamber, along with the γ – subunit and its related ε-and δ-subunits, shapes the rotor of the chemical.
Function of ATP Synthase Beyond Oxidative Phosphorylation
Throughout the long term, proof has gathered in microorganisms and mitochondria that respiratory buildings, including the ATP synthase complex, are coordinated into super-and supramolecular buildings.
Comparable perceptions have been made about photosynthetic buildings in the thylakoid layers of chloroplasts. In the internal layers of mitochondria, dimers of the ATP synthase are shaped by the relationship of their e-subunits in their film areas.
These dimers structure strips, or straight columns, which are confined at the locale of most noteworthy shape along the edges of the cristae.
It is well realized that cardiolipin (diphosphatidylglycerol) is fundamental for the action of a coupled ATP synthase complex.
Cardiolipin atoms have been proposed to tie in the area of a completely trimethylated lysine buildup at position 43 of the c-subunit.
This trimethylated lysine buildup is situated in the headgroup district of the bilayer , and its essence would hamper the limiting of the headgroups of phospholipids, giving destinations to cardiolipin to tie specially.
The ADP–ATP translocase, another plentiful part of the inward layers of mitochondria, additionally contains a trimethyl-lysine buildup, which is likewise in the headgroup area of the layer and would probably have a comparable inclination for restricting cardiolipin atoms.
The suggestion that these trimethyllysine buildups mark cardiolipin-restricting destinations is borne out by atomic elements recreations, and the trimethylated lysine buildup in subunit c is moderated in all vertebrate furthermore, invertebrate ATP synthase edifices that have been inspected, yet not in parasites or microorganisms.
The cardiolipin particles bound to the c8-rings of mitochondrial ATP synthase may balance out the c8- rings to assist them with enduring the rotational force to which they are oppressed.
They may likewise assist with greasing up the pivot of the ring in the film climate. Cardiolipin has been identified additionally in relationship with subunit a.
It is realized that around 75 % of cardiolipin in ox-like heart mitochondria is related with the network pamphlet of the internal layer, and the relationship of cardiolipin with subunit c and the ADP–ATP translocase would contribute to this nearby focus.
In the event that those cardiolipin particles related with ATP synthase buildings were roughly cone-molded, they would in general give the cristae a negative ebb and flow, the inverse to what in particular is noticed.
Consequently, the cardiolipin particles related with ATP synthase are bound to be leveled to the surfaces of the proteins in the layer area of the compound, and their job is probably going to be explicit to the activity of the ATP synthase itself.
ATP Synthase Citations
- Cardiolipin puts the seal on ATP synthase. Proc Natl Acad Sci U S A . 2016 Aug 2;113(31):8568-70.
- Opposite rotation directions in the synthesis and hydrolysis of ATP by the ATP synthase: hints from a subunit asymmetry. J Membr Biol . 2015 Apr;248(2):163-9.
- A novel chloroplast super-complex consisting of the ATP synthase and photosystem I reaction center. PLoS One . 2020 Aug 20;15(8):e0237569.
Share
Similar Post:
-
What is Warburg Effect? an Overview and...
Continue ReadingThe Warburg Effect: How Does it Benefit Cells?
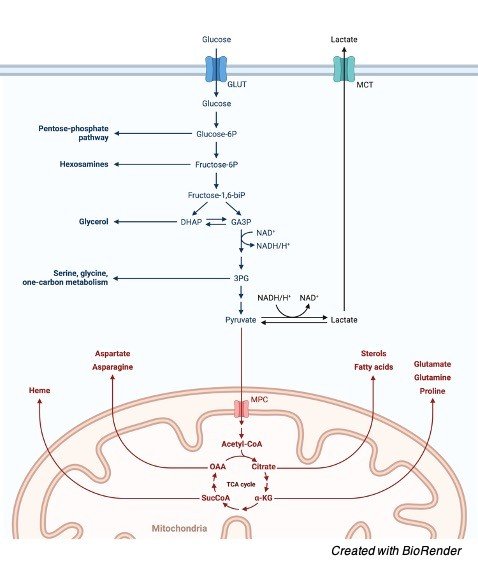
In oncology the Warburg effect is the perception that most cancer cells prevalently produce energy by a high pace of glycolysis followed by lactic acid maturation in the cytosol, not at all like typical cells that complete low paces of glycolysis creating pyruvate that is then used in the mitochondria.
However, this happens in both cancer cells and incendiary cells. Ongoing proof proposes that metabolites themselves can be autogenic by changing cell flagging and obstructing cell separation.
Now cancer related changes in digestion cannot be seen as roundabout reactions to cell multiplication and endurance signals. The evidence fights that changed digestion has accomplished the situation with a center sign of cancer.
Most results of proto-oncogenes and tumor silencer qualities encode segments of cell signal transduction pathways.
Their job in carcinogenesis has customarily been credited to their capacity to control cell cycle and support proliferative flagging while likewise helping cells a-bay development concealment and\or cell passing.
However, proof for an adjusted idea, that the essential capacity of actuating oncogenes and inactivated tumor silencers are to reinvent cell digestion, has kept on working in the course of recent years.
The proof is likewise creating for the proposition the proto-oncogene and tumor silencer articulation is fundamentally developed to direct digestion.

Why Warburg effect?
Quite a bit of this reinventing relies upon using mitochondria as practical biosynthetic organelles. While conventional allosteric guideline surely happens in multiplying cells, solid proof presently exists to help an elective model.
The progressions in metabolic motion happen in essential reaction to development factor flagging, free of changes in ATP and different systems.
The reconstructing of cell digestion towards macromolecular combination is basic to providing enough nucleotides, proteins, and lipids for a cell to twofold its all-out biomass.
Rather than catabolic digestion of separated cells, this anabolic digestion key to cell development and multiplication isn’t centered around expanding ATP yield. Maybe than ATP, multiplying cells are in a lot.
More noteworthy requirement for decreased carbon and diminished nitrogen, just as and NADPH for reductive biosynthetic responses.
The acknowledgment that multiplying cells don’t expand ATP creation through mitochondrial oxidative phosphorylation has added to the proceeding with misinterpretations that multiplying cells, especially cancer cells, don’t use mitochondria.
Is Warburg effect to Compensate Signaling Control?
Actuation of the PI3K/Akt enactment of The PI3K/Akt prompts improved glucose take-up and glycolysis pathway is maybe the most principal normal sore in unconstrained human cancers.
Critical to this acceptance is the expanded glucose transport communicated on the cell surface, actuation of hexokinase to catch glucose intracellularly through phosphorylation, and Akt – incited, PFK-2 to depended allosteric enactment of PFK-1 to submit glucose to glycolytic metabolism.
The PI3K/Akt pathway advances glucose carbon blocks into biosynthetic pathways that depend upon useful mitochondrial digestion. Unsaturated fat digestion, cholesterol, and isoprene amalgamation will require acetyl-CoA.
Mitochondrial acetyl-CoA can’t be straightforwardly sent out to the cytosol however rather should be consolidate with OAA to shape citrate through the movement of the catalyst citrate synthase found in the mitochondria.
Citrate would then be able to be sent out to the cytosol where it very well may be changed over back to acetyl-CoA by ATP-subordinate citrate lyase.
AKT works with this redirection of mitochondrial citrate from the TCA cycle to acetyl-CoA creation by phosphorylated and enacting ATP-dependent citrate lyase.
ATP-subordinate citrate lyase hydrolysis of citrate is urgent to forestall a cytosolic amassing of citrate. Citrate is a significant negative allosteric controller of glycolysis the reconstructing of mitochondrial citrate digestion is a focal part of PI3K/Akt oncogenic movement.
Downstream of P13K/Akt, the very much portrayed cell development controller mTORC1 likewise has numerous effects interwoven with mitochondrial digestion.
A few amino corrosive forerunners are gotten from the transamination of mitochondrial TCA cycle intermediates. OAA can be transaminated to deliver aspartate and αKG can be transaminated to create glutamate, which thusly can be changed over to proline, arginine and glutamine.
An investigation secluding the cell natural outcomes of mTORC1 actuation showed that at SREBP – interceded again lipogenesis is a basic segment of mTORC1 – driven multiplication.
Other significant focuses of mTORC1 initiation, hypoxia – inducible factor 1 (HIF – 1) isn’t basic for mTORC1 driven expansion.
HIF-1 initiation has the extra effect of repressing mitochondrial glucose carbon, to some degree by advancing articulation of pyruvate dehydrogenase kinase-1 to restrain PDH movement.
By redirecting pyruvate into lactate, HIF – 1 squares glucose carbons fuse into mitochondrial citrate, which is basic for lipid combination.
There are two significant focuses to this perception of against proliferative action of HIF-1 apparently the absence of mitochondrial effect seeing might be in cells whose mitochondria are not yet influenced by the change of oncogenesis and irritation.
In these cells instead of oxidative digestion of both glucose and glutamine, these cancers specially perform reductive and carboxylation biosynthetic responses from glutamine carbon.
Myc actuation likewise impacts mitochondrial digestion. Myc advances mitochondrial quality articulation and mitochondrial biogenesis.
Oncogenic Myc has likewise been displayed to advance mitochondrial use of glutamine by the upgraded articulation of glutminase, which deaminates glutamine to glutamate.
Cells communicating oncogenic Myc are glutamine dependent and go through apoptosis when glutamine is removed from the way of culture medium.
While the job of glutamine as a nitrogen benefactor is significant for the multiplication of these cells, there practicality relies upon glutamine as a carbon hotspot for mitochondrial digestion.
As of late, it was seen that the development of tumor xenografts from Myc-communicating B cells can be debilitated by pharmacological hindrance of glutaminase (GLS).
These information give additional proof that reinvented glutamine digestion is basic to the development and endurance of Myc–driven malignancies.
Upstream of Myc, Rho GTPases have likewise been connected to the initiation of GLS and glutamine reliance.
Did proto-oncogenes and tumor silencer’s frame of reference development as segments of metabolic guideline?
The heaviness of the proof to date upholds the idea that the reinventing of cell digestion is an essential and principal part of change coming about because of transformation in proto-oncogenes and tumor silencers.
Why Cancer Cells do Warburg Effect?
Proliferative digestion is intensely reliant upon the reinventing of mitochondria to serve an engineered instead of corruption of job.
Predictable with this speculation initiation of tumor silencer p53 has been demonstrated to be basic for cell endurance following glucose consumption.
Metabolic pressure reaction to p53 to expanded unsaturated fat oxidation and the deficiency of p53 can improve glycolysis and anabolic union from glycolytic intermediates.
Mitochondrial digestion keeps on being basic in cells with metabolic reconstructing emerging from p53 misfortune. It has been shown that denying colon cancer cells of glucose builds the rate at which enacting transformations in Rask developed out enduring clones were better ready to adapt to restricted glucose because of their up guideline of the GLUT-1 carrier.
Expanded glycolytic digestion from enacted Ras doesn’t come from deficient mitochondrial pathways.
In any event, for Ras interceded tumorigenesis, the significance of unblemished mitochondrial oxidation has been affirmed in vivo. Metabolic pathways dynamic in multiplying cells are straightforwardly constrained by flagging pathways and explicit course proteins including known oncogenes and tumor silencer qualities.
The current comprehension of how glycolysis, oxidative phosphorylation, the pentose phosphate pathway, and glutamine digestion are interconnected in multiplying cells.
This metabolic wiring considers both NADPH creation and acetyl-CoA transition to the cytosol for lipid combination.
Key strides in these 5 metabolic pathways can be affected by flagging pathways known to be significant for cell expansion.
Enactment of development factor receptors prompts both tyrosine kinase flagging and PI3K actuation.
By means of AKT, PI3K actuation invigorates glucose take-up and motion through the early piece of glycolysis.
Warburg Effect: a Metabolic Benefit?
Tyrosine kinase flagging adversely manages transition through the late strides of glycolysis, making glycolytic intermediates accessible for macromolecular blend just as supporting NADPH creation.
Myc drives glutamine digestion, which additionally upholds NADPH creation. LKB1/AMPK flagging and p53 decline metabolic motion through glycolysis in light of cell stress.
Diminished glycolytic motion because of LKB/AMPK or p53 might be a versatile reaction to stop proliferative digestion during times of low energy accessibility or oxidative pressure.
Tumor silencers are displayed in red, and oncogenes are in green. Key metabolic pathways are marked in purple with white boxes, and the catalysts controlling basic strides in these pathways are displayed in blue.
A portion of these proteins are applicants as novel helpful focuses in cancer. Malic chemical alludes to NADP-explicit malate dehydrogenase [systematic name (S)- malate:NADP-oxidoreductase (oxaloacetate-decarboxylating)].
Metabolic chemicals can be then again grafted to isoforms that help anabolic development.
Particular articulation of explicit isoforms of metabolic proteins can furnish cancer cells with systems to choose for metabolic options during tumorigenesis.
For instance, multiplying cells all around express the M2 isoform of pyruvate kinase. Pyruvate kinase is the glycolytic protein that changes pap over to pyruvate with attendant age of ATP.
As opposed to the M1 isoform of pyruvate kinase that is overwhelming isoform in most grown-up separated tissue cells, pyruvate kinase M2 to graft variation is the major isoform in undeveloped tissues and an oral cancer cells analyzed today.
The PFK-1/Phosphfructose 2,6 bis phosphatase B3 quality is profoundly communicated in human tumors and has join variations and option grafted isoform of GLS may likewise be critical to the mitochondrial glutamine digestion of tumor cells.
Warburg Effect Citations
- How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat . 2018 May;38:1-11.
- The Warburg Effect, Lactate, and Nearly a Century of Trying to Cure Cancer. Semin Nephrol . 2019 Jul;39(4):380-393.
- Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol . 2021 Mar;599(6):1745-1757.
- Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat . 2015;38(3):117-22.
- The Warburg effect: 80 years on. Biochem Soc Trans . 2016 Oct 15;44(5):1499-1505.
- The Warburg Effect and the Hallmarks of Cancer. Anticancer Agents Med Chem . 2017;17(2):164-170.
- The Warburg effect and drug resistance. Br J Pharmacol . 2016 Mar;173(6):970-9.
- The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol . 2019 Jul;95(7):912-919.
- The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci . 2016 Mar;41(3):211-218.
Share
Similar Post:
-
Adenine: Definition, Structure, and Functions
Continue ReadingAbout Adenine
The specific structure and function of DNA is very much important in sustaining a life.
Adenine is one of the important base pair which is also a part of the DNA. It is also one of the four nucleoid bases which forms a chemical base in the DNA.
The bases are located on one strand and pairs up with thymine which is present on the opposite side on the other strand. This sequence helps in giving up the genetic instructions to the cell.
Adenine also helps in formation of adenosine triphosphate (ATP) molecule which helps in transfer of energy between the cells.
It also paves a way for many chemical reactions to take place.

Features of Adenine
We all know that all the larger substances are made from a smaller building blocks where house or building is made up brick, stone, clay, door, windows etc. Such that DNA is also made up of substances such as sugar and phosphate bonds, nucleotides, base pairs etc.
Adenine is one of the four nucleotides present as a building block of protein.
It is usually represented in genetics with its first letter “A”. It has its own property that when it is that it always pairs up with thymine which is present of its opposite strand.
Adenine is also present in RNA and DNA. Along with it, is also present in ATP molecules. Which helps in restoring and transferring energy to the cells.
Adenine is one of the organic compounds which belongs to the group of purines and it has its own biological importance in the field of genetics and in biochemistry. As it also plays a vital role in maintaining the hereditary of the cell.
Adenine: a Critical Component of Nucleic Acid
Adenine is one of the most important living components of life. It is one of the four nitrogenous bases which are found in DNA as well as in RNA.
DNA and RNA contain specific genetic code for each organism including all the organisms such as humans, plants, fungi, etc. where it helps in giving instructions to the functioning of the cells.
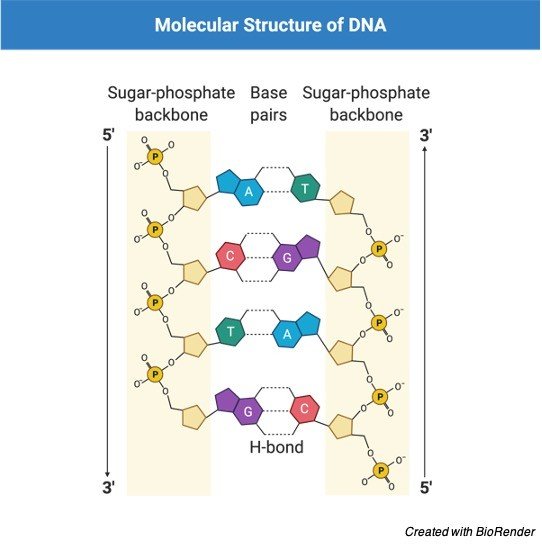
Adenine also helps in stabilizing the portion of nucleic acid in these molecules of DNA or RNA.
In Adenosine Triphosphate adenine helps in storage and transporting oxygen.
Synthesis of Adenine
Adenine is generally formed from the adenylic acid and deoxyadenylic acid by the partial decomposition of ribonucleic acid and deoxyribonucleic acids which may be further separated. These acids are generally known as nucleotides.
These are considered as the phosphodiester of adenosine and deoxyadenosine that are composed of adenine and either ribose or in the form of deoxyribose.
The compounds of adenine is also involved in producing vitamin B12. As it is naturally synthesized by our body and there is no need to intake it in the form of food molecules.
Adenosine triphosphate and other co enzymes are produced which act in conjunction of enzymes.
In some cases, adenine is also known as vitamin B4 because it combines with the ribose sugar to form adenosine, in such case it binds up to three phosphoric acids which results in producing AMP or ADP or ATP.
These adenine groups are important in functioning the vital cellular mechanisms.
Adenine is considered as one of the important nitrogenous bases which are involved in the production of nucleotides. AMP, which is known as adenosine monophosphate is involved in the propagation of many hormonal stimuli by acting as an important secondary messenger.
Adenine also acts as one of the important factors of many co-enzymes. Adenosine is formed, when adenine combines with ribose group or deoxyribose group. But when adenine combines only with ribose group it causes block in the av node of the heart.
Adenosine helps in identifying the rhythm in supraventricular tachycardia affected individuals. Adenine activates when it combines with the ribose and forms an adenosine or in the form of deoxyadenosine.
In the other case when it combines with deoxyribose to form adenosine triphosphate which plays an important role in maintaining the energy functions of the cell.
Chemical Nature and Structure of Adenine
Adenine is usually made up of carbon, nitrogen and hydrogen atoms. Adenine has its chemical formula as C5H5N5.Considering the chemical nature of the adenine, it on combing with ribose and phosphate group forms a nucleotide.

Adenine is one the nucleotide which is present in the group of purines. Where the purine consists of a six membered nitrogen ring fused to a five membered nitrogen ring.
The other group of nucleotides group apart from purine is termed as pyrimidine. Where these pyrimidins has only a single ring which is much less than that of purines.
The adenine and guanine belong to the group go purine and other two bases thymine and cytosine belongs to the group of pyrimidines.
Adenine in DNA
According to the DNA model of Watson and Crick in the double helical structure of DNA, the adenine is present along the sides of the ladder that are composed of phosphate sugar groups. Where the rugs of the ladder are made up of nitrogenous bases that are adenine, thymine, guanine and cytosine where each rug has two bases and they are bonded together by hydrogen bonds.
As of its size and shape purine always pairs up with pyrimidines. Where as Adenine always pairs up with thymine and guanine always pairs up with cytosine or uracil in case of RNA.
These coupling of purine and pyrimidine base pairs are generally called as base pairs. As of DNA adenine has its same importance in RNA also But in such cases, adenine pairs with uracil as thymine is not present.
Citations
Share
Similar Post: