-
Sarcoplasmic Reticulum: Definition, Function, & Examples
Continue ReadingSarcoplasmic Reticulum Definition
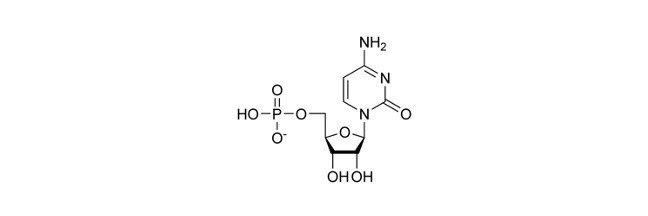
Sarcoplasmic Reticulum is a smooth endoplasmic reticulum type found in striated and smooth muscles that are reserves of calcium ions.
What is Sarcoplasmic Reticulum?
The endoplasmic reticulum (ER) occurs as an interlinked network of flattened sacs in the cytoplasm. The membrane extensions of the organelle are connected to the outer nuclear envelope and also extend into the plasma membrane.
The ER is of two types based on their appearance: the rough endoplasmic reticulum (RER) and the smooth endoplasmic reticulum (SER). RER has ribosomes on their surface while SER lacks them. Sarcoplasmic reticulum refers to specialized SER found in muscle cells.
Sarcoplasmic Reticulum Characteristics
The sarcoplasmic reticulum is present in myocytes as a membrane-bound network of tubules surrounding the myofibrils abounds in the myocyte. Sarcoplasmic Reticulum is located proximal to the transverse tubules in cardiac and skeletal muscle cells. A triad is formed by the union of the sarcolemma that is an extension of the cell membrane and components of Sarcoplasmic Reticulum.
Terminal cisternae are formed by the close association of parts of Sarcoplasmic Reticulum with transverse tubules. It is the primary site responsible for calcium release in the muscle cell.
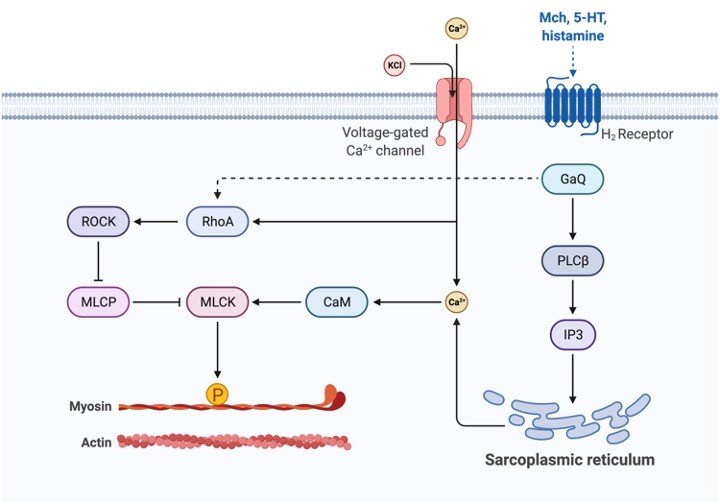
Sarcoplasmic Reticulum has ion channel pumps that help in an influx of calcium ions present on the membrane. These pumps are termed SERCA and consist of 13 subunits. For Sarcoplasmic Reticulum to carry out the role of absorbing calcium ions, it has ion channel pumps in its membrane.
These calcium pumps in the Sarcoplasmic Reticulum are called SERCA and consist of 13 sub-units (M1-M10, N, P, and A). Some subunits are located outside (Eg: N, P, and A) while the rest is present inside the membrane.
ATP binds to the extracellular subunit while Calcium ions bind to the intracellular subunits. SERCA2a is generally found in muscle cells.
Calsequestrin binds to calcium and helps in its storage. This protein is located in terminal cisternae and can bind to 50 Calcium ions at a time. Ryanodine receptors (RyRs) help in the release of calcium ions via Sarcoplasmic Reticulum. The most common receptor is RyR3 present in the brain, while RyR2 is located in cardiac muscles. RyR1 is seen in skeletal muscles.
Sarcoplasmic Reticulum Functions
The Sarcoplasmic Reticulum is crucial for the contraction of myofibrils and functions in the absorption, storage, and release of calcium ions. These ions are released when muscles contract while in the relaxation phase they are absorbed.
Calcium levels need to be regulated to maintain homeostasis. A maintained increase in calcium levels can cause hardening, calcification, and even cell death. The calcium levels are kept constant through Sarcoplasmic Reticulum. As it concentrates the calcium ions inside, it decreases its intracellular levels.
Importance of Sarcoplasmic Reticulum
Calcium ion channel pumps ions into Sarcoplasmic Reticulum utilizing active transport as this is against the concentration gradient. Calcium is taken in when ATP along with 2 calcium ions binds to the cytosolic side of the pump.
The hydrolysis of ATP causes structural alterations of the pump. This leads to the opening of the channel through which the calcium ions pass through into the Sarcoplasmic Reticulum.
The calcium ions are released through the activation of RyR receptors at the terminal cisternae. This causes a surge in ion concentration causes calcium spark that can be spontaneous or evoked.
Calcium-induced calcium release is a mechanism for triggering calcium release. An action potential alters the shape of dihydropyridine receptors resulting in the opening of a calcium ion channel.
The resulting influx leads to the binding of calcium ions to RyR. This receptor is activated on binding with 4 calcium ions and this leads to calcium release. This results in the evoked calcium spark and is observed in the case of smooth and cardiac muscles.
In the case of skeletal muscle, RyR receptors open directly when dihydropyridine receptors change and open, and thus, this occurs without prior flooding of calcium ions. Both kinds of receptors are in proximity to each other, such that any change in dihydropyridine receptor shape triggers RyRs.
In cardiac and smooth muscles, the dihydropyridine receptors are not directly in contact with RyR, and hence calcium ion flooding and binding occurs is prerequisite for the release of calcium.
If no sort of action potentials are required for calcium release, then it is termed a spontaneous calcium spark. This mechanism is dependent on the high calcium ion concentration inside the SR.
The RyR opens by either direct binding of calcium ions or the detachment of calsequestrin from the receptor. If the concentration of calcium ions is low, then calsequestrin inhibits the opening of RyR by binding to it.
Sarcoplasmic Reticulum Citations
- Sarcoplasmic reticulum-mitochondria communication in cardiovascular pathophysiology. Nat Rev Cardiol . 2017 Jun;14(6):342-360.
- The sarcoplasmic reticulum and SERCA: a nexus for muscular adaptive thermogenesis. Appl Physiol Nutr Metab . 2020 Jan;45(1):1-10.
- Sarcoplasmic reticulum and calcium signaling in muscle cells: Homeostasis and disease. Int Rev Cell Mol Biol . 2020;350:197-264.
- Images created with BioRender.com
Share
Similar Post:
-

Trehalose: Definition, Function, & Examples
Continue ReadingTrehalose Definition
Trehalose consists of α-glucose molecules that form disaccharides and is a source of energy for organisms like fungi, plants, invertebrates, and bacteria.
What is Trehalose?
Carbohydrates are a sub-class of biomolecules that is categorized depending on the number of saccharide constituents. When 2 monosaccharides are linked by a glycosidic linkage they form a disaccharide i.e., a carbohydrate an example of a disaccharide is trehalose.
Trehalose History
It was H. A. L. Wiggers in 1832 who discovered trehalose in ergot of rye. But the name of this disaccharide was given in 1859 by Marcellin Berthelot based on its source trehala, which was extracted from the weevil’s pupal case.
Trehalose Properties
The monosaccharides in trehalose are linked by 1-1 α-glycosidic linkage. It has a white crystalline appearance and it is also known as α-D-glucopyranosyl-(1→1)-α-D-glucopyranoside and has a formula C12H22O11.
In terms of its components i.e., saccharides it is similar to maltose as it is comprised of 2 glucose units. But, maltose differs with respect to the glycosidic bond, it has α-1→4 glycosidic linkages.
Unlike trehalose where this bond occurs between C-1 of either glucose, on maltose the linkage occurs between C-4 and C-1. If the glycosidic linkage between 2 glucose units is β-(1→4), then they form cellobiose whereas if the linkage is α-(1→6) then isomaltose is formed. Unlike trehalose, maltose is a reducing sugar.
The closed ring structure in trehalose is due to the glycosidic bond between glucose units. Due to this, it is resistant to acid hydrolysis as the aldehyde or the ketone ends cannot bond with lysine or arginine of proteins.
This disaccharide is also stable at high temperatures and in acidic conditions. As it is an energy source and also has high retention trait organisms can endure long dry or freezing situations.
Trehalose Biosynthesis
The only anomer of trehalose is α,α-trehalose, and is naturally utilized by different organisms as an energy source. These organisms have inherent pathways and enzymes required to produce this disaccharide biosynthetically. One general metabolic pathway requires a trehalose-phosphate synthase enzyme.
From UDP-glucose the glucose unit is transferred to glucose-6-phosphate and the reaction is catalyzed by trehalose-phosphate synthase to produce threlaose-6-phosphate and UDP. Then, trehalose-P phosphatase catalyzes the dephosphorylation of trehaloses-phosphate.
Trehalose via trehalase enzyme is broken down into glucose units that provide energy in organisms like insects for flight. In the case of higher animals including humans, this reaction is catalyzed by α-glucosidase trehalase that is present in the brush border of cells of the intestinal mucosa.
Biological Importance of Trehalose
This natural disaccharide is biosynthesized in organisms like invertebrates, fungi, and plants. In these organisms, it acts as both the source of carbon and energy. It not only provides a fast energy source but also forms a structural entity in the cell walls of certain organisms and acts as an osmoprotectant.
Plants like Selaginella can survive long periods of dehydration, desiccation, oxidative stress, and heat by utilizing trehalose. During the dry spells it shrinks into a brown ball-like shape but on contact with moisture resumes growth. This phenomenon of resurrection is referred to as poikilohydric.
In omnivores and herbivores, sources like mushrooms, shellfish, and starchy food can deliver trehalose. This is broken down by trehalose that attacks the glycosidic linkage.
It can also be produced synthetically for commercial purposes to produce drugs, food, frozen foods, and cosmetics. In frozen foods like ice cream, this is added as an ingredient to decrease the freezing point of food.
It can also be used as an artificial sweetener as it does not cause a spike in glucose levels in the blood. It also promotes the growth of gut microbes like Clostridium difficile so consumption beyond the required amount is not advised.
Certain toxins are also released as a result of trehalose metabolism. Inborn error of metabolism Trehalase deficiency is a rare metabolic condition in the case of humans where the enzyme is not sufficiently produced and as a result, it accumulates.
It can lead to diarrhea and abdominal discomfort when ingesting dietary trehalose. This deficiency can result due to mutation in the TREH gene.
Trehalose Citations
Share
Similar Post:
-
Polyols: Definition, Function, & Examples
Continue ReadingPolyols
Polyols are primarily organic alcohol that has multiple hydroxyl groups. The name polyol is comprised of 2 terms, “poly” refers to multiple and –“ol” indicates alcohol group.
What are Polyols?
Polyols like other organic compounds comprise C-C and C-H covalent chemical bonds. Sugar alcohols are a group of polyols that are derived from sugars produced naturally or synthetically. Even though they are formed by the hydrogenation of sugars, they are not true sugars.
Sugars are comprised of monosaccharides and disaccharides having a formula Cn (H2O) n, where n may range from 3 – 7.
The group sugar alcohols are similar in structure to sugars except for the fact that they have additional hydroxyl groups and have the formula (CHOH)nH2. They are less sweet and can act as an energy source.
Polyols Pathway
Glucose is converted into fructose in the polyol pathway through a biological process. In this pathway, glucose is first reduced to polyol sorbitol that is oxidized to fructose. The first reaction is catalyzed by aldose reductase whereas the oxidation of sorbitol occurs in presence of the enzyme sorbitol dehydrogenase.
This pathway also employs cofactors, NADPH and NAD+. Dysfunctions in this pathway can lead to microvascular damage to kidney cells, retina, or nerves that are insulin-independent leading to type-II diabetes complications.
If glucose has not been phosphorylated by hexokinase then instead of the glycolytic pathway, it enters the polyol pathway where it is converted to fructose.
This occurs when there is a surplus of glucose due to which enzyme hexokinase becomes saturated and the excess glucose is diverted to the polyol pathway instead. Here it is reduced sorbitol and utilizes NADPH and NAD+.
These co-factors become less available for other significant pathways and metabolic reactions like glutathione and nitric oxide production. It also increases free radical oxygen species which can be damaged cells.
Biological Importance of Polyols
The most important function of polyols is to provide an energy source and it is involved in the polyol pathway where glucose is reduced. In this pathway, excess glucose is reduced to fructose which is required for processes like glycation and fructolysis.
Natural polyols occur naturally in fruits like apples, prunes, peaches, and in prunes, in forms like sugar alcohol sorbitol. Another naturally occurring polyol is found in bacterial cell walls as sugar alcohol ribitol.
Polyols may also act to maintain high intracellular osmolality as osmolytes in the kidney cells of higher organisms; some also act as antifreeze molecules.
Artificially polyols can be synthesized starch and sugar. They are used commercially as an alternative for sugar or as additives. They are employed in food products that are categorized as having no sugar content or low calorie. These sugar sorbitols except erythritol provide about 2.4 kilocalories/g.
They are not labeled as essential nutrients. But for diabetic patients, it can help them in regulating glucose levels in the blood. They can also act as a substitute for sucrose that is also a natural sugar. It is synthesized by plants like beets and sugarcane.
It is extracted from these sources and commercially processed as sugars utilized in various preparations. But unlike polyols, it is an essential carbohydrate that provides both fructose and glucose.
One of their disadvantages is that it has a high GI index of 65 when compared to sugar alcohols that have a GI of less than 10.2. GI of glucose is 100, while fructose has a GI of 25.
A high glycemic index is not preferred as it means that the substance can cause a substantial peak in blood glucose levels as in conditions of obesity and diabetes mellitus.
Sugar alcohols with a low GI are preferred and they do not promote tooth decay as they are not metabolized by mouth oral microbes. They are used commercially in the production of medicinal syrups, lozenges, and toothpaste as they do not produce any sort of acids that might trigger tooth decay.
One of their downsides is that they are poorly digestible which can lead to irritation and increased fermentation by gut microbe leading to bloating, flatulence, and diarrhea. These conditions can be triggered even in smaller amounts for sensitive individuals.
Polyols Examples
Sugar alcohols class includes maltitol, sorbitol, xylitol, isomalt, erythritol, glycerol, lactitol, hydrogenated starch hydrolysates, and mannitol.
The most common polyol is sorbitol that is found naturally in fruits like pears. It can also be produced artificially for commercial purposes for the production of sugar-free food products like sugar-free ice cream.
Maltitol is another commonly used alternative polyol that can replace sugars that are employed in manufacturing sugar-free products like baked goods. Another polyol that is commonly used in candies is isomalt that is a disaccharide.
Polyols Citations
- Review on the Impact of Polyols on the Properties of Bio-Based Polyesters. Polymers (Basel) . 2020 Dec 12;12(12):2969.
- A review of polyols – biotechnological production, food applications, regulation, labeling and health effects. Crit Rev Food Sci Nutr . 2020;60(12):2034-2051.
- A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv Nutr . 2017 Jul 14;8(4):587-596.
Share
Similar Post:
-

Thymine: Definition, Structure, & Function
Continue ReadingThymine Definition
Thymine is a pyrimidine base having the formula C5H6N2O2 that pairs complementary with adenine via 2 hydrogen bonds in the DNA molecule.
What is Thymine?
Nucleotides form the fundamental monomers that comprise the polymer of nucleic acids like DNA. Each nucleotide is formed of 3 sub-units: pentose sugar, phosphoric acid, and a nitrogenous base.
Nitrogenous bases include There are five nucleobases that serve as fundamental units of the genetic code: thymine, adenine, cytosine, guanine, and uracil. These nucleobases are grouped into purines and pyrimidines.
Thymine like other pyrimidines has a heterocyclic aromatic ring and has a molar mass of 126.115 g/mol. It has a melting point of 316- 317 °C. It forms the component of occurring as a component of a nucleoside or a nucleotide.
It forms the part of nucleobases in the case of DNA but it is absent in RNA and uracil is present instead. Uracil and thymine both pair complementary with adenine.
Thymine vs Uracil
Thymine, cytosine, and uracil are pyrimidine nucleobases. Thymine is distinct from other pyrimidine bases in having in 5th position a methyl group and 2 keto groups at positions 2 and 4 in the heterocyclic ring. Thymine pairs with adenine with 2 hydrogen bonds.
Uracil has a similar structure to thymine and it only differs in terms of a methyl group at the 5th position in the heterocyclic ring. One of the possible explanations why DNA has thymine instead of uracil can be due to stability.
Cytosine another pyrimidine gets readily deaminated into uracil by losing an amine group. This defect is sensed and corrected by repair systems in DNA before a mutation could occur.
If uracil was present as a pyrimidine base in DNA, the repair system may fail to differentiate between deaminated cytosine turned uracil and the original uracil.
Cytosine in the 2nd position has a keto group and an amine group at position 4 in the pyrimidine ring and the formula C4H5N3O. Cytosine complementary bonds with guanine via 3 hydrogen bonds in both RNA and DNA.
Biosynthesis of Thymine
Thymine biosynthesis, like other pyrimidines, is initiated by the formation of carbamoyl phosphate. It forms in a reaction catalyzed by carbamoyl phosphate synthetase that involves ATP, glutamine, water molecules, and bicarbonate.
In a series of steps, the carbamoyl phosphate is first converted into carbamoyl aspartate that then yields dihydroorotate. It undergoes oxidation to yield orotate that then gets converted into orotidine-5-monophosphate (OMP).
OMP on decarboxylation generates uridine monophosphate (UMP). Finally, down the pathway, uridine triphosphate (UTP) and uridine diphosphate (UDP) are produced.
Uridine in a reaction catalyzed by ribonucleotide reductase gets reduced to deoxyuridine that is methylated to form thymidine in presence of thymidylate synthase.
Thymine on being attached to a deoxyribose sugar is known as deoxythymidine. Further, when 3 phosphoric acid groups are attached to it, then it becomes deoxythymidine triphosphate (dTTP), one of the monomeric nucleotide units.
Thymine on degradation gets converted to β-aminoisobutyrate that then enters the Citric acid cycle. Thymine may also be recycled in a salvage pathway. Thymine on reaction deoxyribose-1-phosphate and enzyme thymidine phosphorylase gets first converted into thymidine. That is then converted in presence of enzyme nucleoside kinase to thymidine monophosphate.
Thymine Dimers
Mutations are alterations in the nucleotide sequences that may cause a change in the functioning of the gene. Thymine dimers are common mutations in the DNA that involve adjacent thymines.
Thymine dimers formed in presence of UV light and can result in kinks in the DNA structure. Such mutations are repaired by base excision repair by utilizing the undamaged DNA strand as a template to replace the mutated region of the other strand.
Thymine Function
Thymine is one of the primary nucleobases in DNA that comprise the genetic code. The nucleotide sequence codes for a particular protein having a specific amino acid sequence.
Thymine Citations
Share
Similar Post:
-

Uracil: Definition, Structure, and Function
Continue ReadingWhat is Uracil?
It is an organic primary nucleobase occurring only in RNA with a formula of C4H4N2O2 and pairs complementary with adenine. It has a molar mass of 112.08676 g/mol.
The monomeric nucleotides form polymers of nucleic acid-like RNA and DNA. Each nucleotide is comprised of nucleobase, a pentose sugar, and a phosphoric.
Guanine, Adenine, Cytosine, Uracil, and Thymine are the 5 fundamental nucleobases that comprise basic units of genetic code. Nucleobases can be pyrimidine or purine. Uracil is an example of a pyrimidine base.
Uracil Properties
Uracil like other pyrimidines is an aromatic heterocyclic compound with a pyrimidine ring that comprises alternating nitrogen and carbon atoms. Uracil has a melting point of 335 °C. It is a part of both nucleosides and a nucleotide. It under normal conditions is not seen in DNA but is present in RNA replacing thymine.
Uracil vs Thymine
Pyrimidine nucleobases include thymine, cytosine, and uracil. Structurally uracil and thymine are similar except for a methyl group present at position 5 in the aromatic ring in thymine. Through complementary base pairing, it bonds with adenine via 2 hydrogen bonds.
In DNA thymine is present, it at 2nd and 4th position consists of keto groups, and at 5th position a methyl group in the ring. Thymine pairs with adenine in DNA with the hep of hydrogen bonds.
Uracil is absent in DNA as it can destabilize DNA and repair systems in DNA cannot distinguish between deaminated cytosine that turns into uracil and the original uracil. This can lead to point mutations if not repaired.
Cytosine is structurally different from uracil in having an amine group at position 4 and a keto group at position 2 in its heterocyclic ring and has a formula of C4H5N3O. It occurs in both types of nucleic acids and it pairs up with guanine.
Biosynthesis of Uracil
Pyrimidine formation is initiated with the compound carbamoyl phosphate that is formed in a reaction catalyzed by enzyme carbamoyl phosphate synthetase. Further the following series of reaction occur:
carbamoyl phosphate>> carbamoyl asparatate>> dihydroorate>> orotate>> OMP. Orotidine-5-monophosphate or OMP yields uridine monophosphate on decarboxylation.
Ultimately by the activity of kinases and dephosphorylation of ATPs, at the end of the pathway, UDP and UTP are formed. UTP is aminated in the presence of the enzyme CTP synthetase to produce CTP.
Uracil when attached to a deoxyribose sugar is called uridine and when phosphorylated with 3 phosphoric acid groups, it becomes uridine triphosphate or UTP. UTP constitutes one of the primary monomeric building units that comprise RNA.
Degradation of uracil occurs in steps. It is converted first into β-alanine that is then degraded into malonyl-CoA. This can be utilized in the synthesis of fatty acids. If it is degraded further it will lead to the formation of ammonium along with CO2 and water. This ammonium is further processed in the urea cycle.
Uracil is recycled via the salvage pathway. For example, it reacts with ribose-1-phosphate in presence of the enzyme uridine phosphorylase to produce UMP.
Biological Function of Uracil
Uracil aids in the formation of nucleotides like UDP, UMP, and UTP. The naming of nucleotides depends on the number of phosphate groups attached to them. The nucleotide UDP is important for glycogenesis. UDP is formed when glucose-1-phosphate combines with UTP.
Glycogen is synthesized when enzyme glycogen synthase combining individual UDP-glucose units. As these UDP-glucose are added, UDP is cleaved. Therefore, UDP aids in the formation of glycogen from glucose in the liver and muscle cells.
UTP is important in transcription as it is the direct precursor of RNA. Uracil aids in the formation of nucleotides like UDP, UMP, and UTP. The naming of nucleotides depends on the number of phosphate groups attached to them.
The nucleotide UDP is important for glycogenesis. UDP is formed when glucose-1-phosphate combines with UTP. Glycogen is synthesized when enzyme glycogen synthase combining individual UDP-glucose units.
As these UDP-glucose are added, UDP is cleaved. Therefore, UDP aids in the formation of glycogen from glucose in the liver and muscle cells. UTP is important in transcription as it is the direct precursor of RNA.
Uracil Citations
- In search of uracil derivatives as bioactive agents. Uracils and fused uracils: Synthesis, biological activity and applications. Eur J Med Chem . 2015 Jun 5;97:582-611.
- The synthesis and antituberculosis activity of 5-alkynyl uracil derivatives. Bioorg Med Chem Lett . 2020 Aug 15;30(16):127351.
- Therapeutic potential of uracil and its derivatives in countering pathogenic and physiological disorders. Eur J Med Chem . 2020 Dec 1;207:112801.
Share
Similar Post:
-
Cellular Respiration: Definition, Types, and Examples
Continue ReadingCellular Respiration Definition
For the production of ATP, molecules like glucose are oxidized, this is called as Respiration. It involves 3 stages and occurs at various positions within the cell. In aerobic as well as anaerobic condition, respiration can take place.
Cellular Respiration Steps
i. Glycolysis
In Glycolysis, a 6C hexose undergoes few steps and gets disintegrated into a 3C compound called the pyruvate. This process takes place in the cytoplasm and requires 2 ATP for this process and obtains 2 ATP out of the process. The hydrogen atoms from the glucose are taken by the cytochrome system.
Glucose molecule transforms into pyruvic acid is the last step in anaerobic respiration, however in aerobic condition it is further degraded into Krebs cycle compound. However, when not present it can be transformed to lactic acid.
ii. Krebs Cycle
From a single glucose molecule, more ATP can be created, in aerobic conditions. The pyruvic acid from the cytosol in glycolysis is moved to mitochondria, an organelle which has large area and is somewhat sausage shape and respiration will take place over here.
Pyruvic acid in aerobic condition it is further degraded into Kreb cycle 2C compound. Now the 2C compound will further attach to a 4C compound which is a part of the cycle.
In aerobic respiration, oxygen binds with carbon compound and CO2 is liberated. Thus, this is why animals liberate out CO2. Carbon compounds are oxidized by the enzymes and the hydrogen atom are given to the cytochrome system.
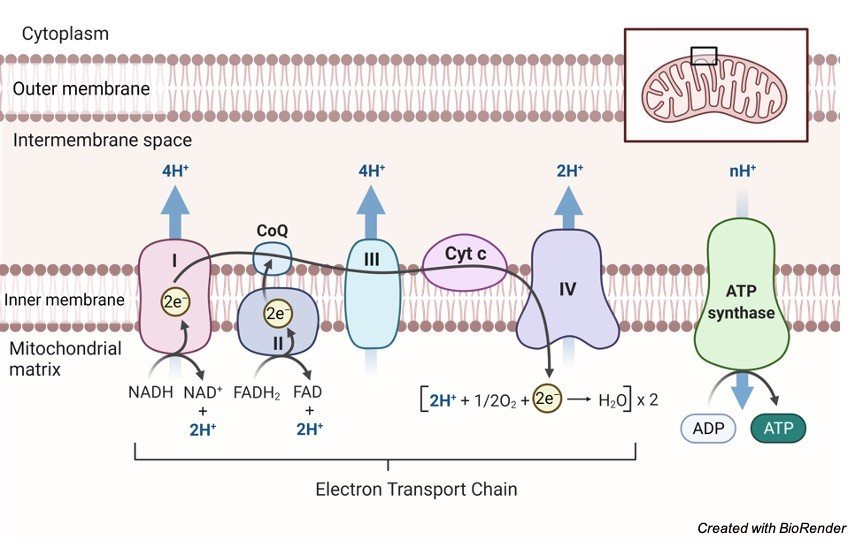
iii. Cytochrome System
The hydrogen carrier system, another name for cytochrome system, is the place where the hydrogen atoms are dispatched from kreb cycle and glycolysis. In the mitochondria is the cristae, where extremely small particles are present in the outer layer. The atoms are collected by the hydrogen acceptors.
The working of cytochrome system is as follows: where the previous coenzymes are consigned to the other coenzymes, which gets oxidized and liberates energy and hydrogen, these hydrogen will attach with the oxygen atoms of the aerobic respiration and will result in the formation of water molecule.
From one single molecule of glucose, 38ATP is gained in aerobic respiration. Glucose is also obtained from the food we consume, which can be utilized during respiration, however the mechanism differs. Through respiration plants also obtain energy.
Cellular Respiration Citations
Share
Similar Post:
-

Top 10 Best Online Criminology Graduate Program
Continue ReadingWhat is Criminology Graduate Program?
Criminology Graduate Program studies the Interplay between Society and Law Criminology programs examine the many laws and institutions that make up the criminal justice system, as well as social and psychological aspects, including the origins and effects of crime. In criminology, you can work in a wide variety of fields, including law enforcement and community and social services.
Protective and social services occupations pay well and have higher job growth rates than the average occupation. Graduates with a bachelor’s degree may be eligible for more advanced roles within these organisations.
What Can I Expect from an Online Criminology Graduate Program?
Most Criminology Graduate Program require a rating of one hundred twenty credit score factors and take four years to finish. Students generally have very non-public decision-making, important thinking, and problem-fixing skills.
Criminology Graduate Program packages function with a particular curriculum and options of hobby that would cause precise careers. Concentrations in regulation, for example, can cause careers withinside the jail system, whilst concentrations in psychology attention on studying the human beings and situations withinside the system.
Some mergers, in addition to beneath preliminary protection, can cause careers mainly organizations. Some criminology kits paintings on elements that assist university college students meet licensing or education requirements.
For example, college students may additionally need to finish an enormous variety of running hours to qualify for regulation enforcement education packages and safety certifications. While diploma packages in era typically provide extra specialized education withinside the sciences, diploma packages in literature provide complete education.
Prospective police officers and detectives frequently discover a better fee in a BA. However, employers take delivery of all diplomas, and essential guides typically do now no longer fall beneath those options.
Online Criminology Graduate Program Route Guide
Although all guides are unique, the subsequent listing describes the center guides that seem often in Online Criminology Graduate Programs guides.
Cybercrime: This route examines the ancient evolution of cybercrime in addition to felony and systemic responses. The college students examine unique sorts of assaults and techniques for detection, prevention and felony action. Other subjects consist of cyber forensics and regulation enforcement for cyber criminals.
Global Terrorism: This route covers terrorism across the world, inclusive of its definition, reasons and responses. The college students evaluate country wide and global terrorism and observe how country wide safety goals to save you and thwart assaults.
Mental Health and Justice: In this route, college students observe the interface among intellectual fitness and the felony machine. Students speak how intellectual fitness troubles can result in crook interest and the way the felony machine defines intellectual fitness and treats humans with intellectual infection troubles. fitness concerns.Students additionally examine network intellectual fitness packages and the way they supplement the felony machine.
Criminal Psychology: This route examines crook conduct and feasible mental reasons of crook interest. Students have a look at how the felony machine and regulation enforcement groups use psychology to research criminals and crook interest.
Social Inequality: This route examines how social inequality influences crook conduct and the remedy of perpetrators and sufferers withinside the judiciary. The college students observe race, gender and sexual orientation in addition to the remedy of contributors of various organizations differently. through regulation enforcement groups and in felony proceedings.
What Can I Do With Online Criminology Graduate Program?
Graduates with Online Criminology Graduate Program degree qualify for lots of professions. Police officer and detective jobs are a number of the best-regarded and maximum profitable positions withinside the field.
While a Criminology Graduate Degree can assist graduates meet coaching desires for criminology professions, a few majors require extra training. Also extra specialised positions along with B. Forensic technicians and emergency manipulate officials can also additionally require unique interest and / or training.
Careers as correctional officials and employees investigators not require a diploma, however candidates with a bachelor’s diploma regularly have an advantage. Find out what to do with a Online Criminology Graduate Degree.
Policeman: Policemen guard humans and assets from crime and criminals. They do behavior investigations, put together instances and patrol their regions of responsibility. According to the Bureau of Labor Statistics (BLS), maximum of those experts paintings for nearby governments as patrol officials.
Probation Officials: Probation officials offer social offerings as a part of the rehabilitation system for offenders and offenders. They examine the humans they take care of and decide which sorts of offerings and aid are maximum useful and effective.
Correctional Officials: Correctional officials running in prisons and prisons. You are answerable for the protection of humans and assets inside facilities, put in force guidelines and save you disruptions.
Forensic Technician: Forensic Technician Collect and examine proof as a part of a crook investigation. They file crime scene observations, have a look at microscopic or chemical proof, and offer statistics to investigators. According to the BLS, maximum of those experts paintings for nearby governments, kingdom governments and laboratories.
Private Investigators and Detectives Investigators: Paintings for people and agencies investigating economic and private activities, engaging in surveillance, and accumulating proof of crime and misconduct. Researchers ought to follow the legal guidelines of the jurisdictions wherein they do their paintings. You might also additionally want a license.
Top 10 Best Universities Offering Online Criminology Graduate Program
UF Online offers a Criminology Graduate Program that teaches students the theories and methodologies for studying the intersections of crime, law, and society. As a criminal investigator, probation officer and advisor on substance abuse and behavioral disorders.
The UF Criminology Graduate Program requires 120 credits and lasts four years. Core requirements include courses in advanced principles of criminal justice, law and judicial process, and law and society.
Each candidate must have a high school diploma (or equivalent), a cumulative “C” average, competitive SAT or ACT scores, and good behavior. The transfer applicant must have at least a grade point average of 2.5 and have completed the prerequisite courses as an introduction to statistics. UF Online is regionally accredited by the Commission on Universities of the Southern Association of Colleges and Schools.
For those who want to acquire a Bachelor of Science degree in a Florida public institution and a bachelor’s degree, USF offers a Bachelor of Applied Science degree with a concentration in criminal justice. The legal method prepares students for law enforcement, incarceration, and corrections studies or professional training.
In addition to the associate degree, this bachelor’s degree in applied sciences with a concentration in criminal justice necessitates 60 hours every week. Theories of criminal conduct and a survey of the criminal justice system are among the topics covered in focus courses.
Each applicant interested in Criminology Graduate Program must have an AS degree with a minimum GPA of 2.0 from a Florida State or Community College. Enrolling in a Bachelor of Applied Science programme allows you to transfer up to 60 credits from your AS degree.
UWW offers an undergraduate law enforcement program leading to a Criminology Graduate Program for law enforcement officers three years or older who already hold a personal degree. The program combines credits from prior learning, independent learning, online learning and traditional classroom courses.
Graduates can complete the criminology course of the UWW in three years, compulsory subjects are an introduction to criminology, criminal investigation, criminological theory and forensic documentation.
Each applicant must complete an initial Expression of Interest form on the program website. UWW is regionally accredited by the Commission for Higher Education.
Students who get an online Criminology Graduate Program and criminal justice from Florida State University are prepared to work as child care providers, victim attorneys, private investigators, probation officers, and social administrators. A high school diploma or a legal degree can also be used.
The online Criminology Graduate Program requires completion of all general education credits and 36 credits in specific specialised courses. The compulsory courses include criminal justice fundamentals, white-collar crime, black men’s sociology, and crimes against humanity.
Each student must obtain an Associate of Arts degree from a Florida university or meet FSU liberal studies requirements, complete 36 credits in criminology courses with a “C” or better in three core courses, and maintain a 2.0 GPA in order to graduate. The University Commission of the Southern Association of Colleges and Schools has accredited Florida State University as a regional institution.
LeTourneau University Location Longview, TX LETU gives a bachelor of technology in crook justice that offers college students with an knowledge of crook conduct and fine strategies for struggle resolution, which includes restorative justice and opportunity conduct amendment incentives. Enrollees can pursue online and on-campus formats.
The crook justice diploma calls for one hundred twenty credit, which includes 36 credit of main coursework. Students can select from 3 concentrations: crook justice management, human trafficking, and hometown security. Required main guides encompass crook law, justice and human rights, and police and the community.
University necessities encompass theology and vocation guides. Each applicant should maintain an excessive college diploma (or equivalent), a minimal 2 five GPA, and a minimal SAT rating of 1030. LETU is locally permitted with the aid of using the Southern Association of Colleges and Schools Commission on Colleges.
Missouri State University Location Springfield, MO MSU gives Bachelor of Science and Bachelor of Science levels in criminology and the arts with levels in criminology. College students are required to claim their most important earlier than finishing 60 credit at MSU. The take a look at of criminology calls for forty five credit within-side the primary necessities similarly to the overall academic necessities.
Core topics are juvenile crook regulation, crook process regulation in addition to crook regulation and courts. MSU enrolls college students on a sliding scale primarily based totally on their elegance rank and GPA that determines what minimal check ratings they have to have at the SAT or ACT.
The better a candidate’s elegance rank and common grade, the decrease the check grade. The MSU is domestically accepted with the aid of using the University Commission.
Saint Mary of the Woods, IN SMWC gives a bachelor of technology in criminology that prepares people for careers in regulation enforcement or the crook justice system. The application presents a social technology know-how of crime and crook behavior, along side a stable basis in liberal arts.
The bachelor of technology application calls for 36 credit of most important coursework in criminology, similarly to standard training requirements. Major middle publications encompass juvenile justice, corrections, and crook interviewing and investigation. Students can pick out from concentrations in psychology or sociology.
Each applicant has to have own an excessive college diploma (or equivalent) and put up their transcripts. Test scores, along with the SAT or ACT, aren’t required for admission however are encouraged. SMWC is locally approved via way of means of the Higher Learning Commission.
University of North Carolina at Wilmington Location Wilmington, NC gives an online bachelor of arts in criminology with concentrations in crook justice, criminology, and public criminology. Enrollees can whole the primary concentrations totally online, at the same time as the general public criminology attention can also additionally require a few in-individual classes.
The Criminology Graduate Program application calls for 39 credit of most important coursework, further to all standard training and college requirements. Core publications encompass criminology, techniques of social research, and sociological information evaluation and interpretation.
Students should additionally whole seminars of their selected vicinity of attention. Each applicant should own a excessive college diploma (or equivalent) and a minimal 2 five GPA. Transfer college students should have 24 credit of transferable college-degree coursework. UNCW is domestically authorized via way of means of the Southern Association of Colleges and Schools Commission on Colleges.
Regis University Denver, CO Regis offers Criminology Graduate Program that examines crime as a social phenomenon and emphasizes a sociological method to studying the social, political, and environmental elements that impact crime. Regis additionally gives a blended bachelor’s to master’s application in criminology for folks that plan to pursue similarly graduate research and desire to shop time.
The Criminology Graduate Program calls for a hundred and twenty credit and takes 4 years to complete. Major coursework consists of creation to forensic technology, crime analysis, psychology of crime, and own circle of relative violence. Program electives provide publications on subjects of teenager’s violence and delinquency, hometown security, and animal exploitation.
Each applicant ought to own an excessive faculty diploma (or equivalent) and put up all preceding transcripts. Regis is domestically authorized with the aid of using the Higher Learning Commission.
Arizona State University is certainly considered one among the most important colleges withinside the U.S., presently enrolling almost 75,000 college students. This main studies college gives greater than three hundred online degrees, which include online Criminology Graduate Program in criminology and crook justice.
Students on this BS application discover the reasons and outcomes of crime, in addition to the jobs of companies accountable for coping with crime. The 120-credit score curriculum covers subjects including creation to policing, courts and sentencing, and statistical analysis.
Graduates can pursue quite a few profession paths, which include positions as correctional officers, forensic specialists, and fraud investigators. To apply, potential college students should preserve a minimal 3.zero excessive faculty GPA, rank withinside the pinnacle 25% in their graduating class, or meet minimal SAT or ACT rating requirements.
Share
Similar Post:
-
Phosphorylation: Definition, Types, and Examples
Continue ReadingPhosphorylation Definition
Phosphorylation is the transfer of a phosphoryl group from a donor to a receiver molecule.
Phosphorylation is a biological process that involves the addition of a phosphate molecule to an organic substance such as glucose or adenosine diphosphate (ADP).
In the latter case, the addition of the phosphate group to transforms ADP to adenosine triphosphate (ATP), a crucial molecule that provides energy for a variety of functions in living cells, including nerve impulse transmission and muscular contraction.
The addition of a phosphate molecule to an organic substance is the easy response.
What is Phosphorylation?
Phosphorylation is a crucial biological step in which phosphate molecules are added to an organic component to make it useful for a variety of activities in a live organism.
Another similar term is “phosphorylate,” which refers to the addition of a phosphoryl group to an organic molecule.
The phosphorylation reaction is very important in biology since it is required for numerous biological processes including as apoptosis, inflammation, metabolic control, subcellular transport, and proliferation.
The transfer of phosphate molecules to a protein is known as phosphorylation in biology. In a living creature, this transfer prepares proteins for specific functions.
In humans, phosphorylation takes place on the side chains of three amino acids: (1) tyrosine (an amino acid produced in the body of living creatures from another amino acid called “phenylalanine”), (2) serine, and (3) threonine. However, histidine phosphorylation has also been shown in certain investigations.
Tyrosine, serine, and threonine are the amino acids that can be phosphorylated.
Phosphorylation is a reversible process, which means it may add and remove phosphate molecules. Kinases are the enzymes that are responsible for adding phosphate groups to proteins. The enzymes that remove these phosphate groups are known as “phosphatases.”
Purpose of Phosphorylation
Phosphorylation plays a crucial role during a sort of biological processes. It plays a role in the cell’s control of protein activities and signal transmission. Cell growth, signal transmission, cell development, protein synthesis, and cell division are all aided by it.
transmission, cell development, protein synthesis, and cell division are all aided by it. The reaction of phosphorylation is reversible. Phosphorylation and dephosphorylation (opposite of phosphorylation) activate and deactivate the majority of enzymes and receptors, respectively. Phosphorylation of enzymes acts as an on/off switch, changing the activity or function of the enzyme.
The phosphate molecule is a critical component of biomolecules. The researchers discovered that a huge number of metabolites (metabolite intermediates or end products) are phosphorylated in cells, resulting in the production of phosphates.
Initially, two scientists, Thomas Rall and Earl Sutherland, were doing study on the link between glucagon and epinephrine and therefore the liver enzyme glycogen phosphorylase.
The hormones in question were discovered to be involved in the synthesis of 3,5-cyclic adenosine monophosphate. This chemical was discovered to function inside a cell to alter the activity of phosphorylase. As a result, 3,5-cyclic adenosine monophosphate is a molecule that acts as a messenger for hormonal signals.
The phosphorylation of tyrosine regulates protein activity. Tyrosine is an amino acid that our bodies make from phenylalanine, another amino acid. The phosphorylation of the regulating tyrosine causes enzymatic activity-related modifications in the substrate. Phosphorylation can also be used for the following objectives;
1. Protein dissection
2. Control of Enzyme Inhibition
3. Glycolysis activity
4. Protein-protein communication
5. Controls energy-intensive chemical processes.
The Mechanism of Phosphorylation
Protein phosphorylation takes place on the side chains of three different amino acids, notably serine, threonine, and tyrosine, which are phosphorylated reversibly in eukaryotic cells.
The terminal phosphate group is targeted by the nucleophilic (–OH) group in these amino acids. The nucleophilic (–OH) group in these amino acids binds to the phosphate terminal group (-PO32-) and is transferred from the phosphate group to the amino acid side chain by the usual phosphoryl donor adenosine triphosphate (ATP), commonly known as ATP phosphorylation.
Magnesium (Mg2+) facilitates this transition, which lowers the phosphoryl transmission threshold in the γ- and ß-phosphate groups through the nucleophilic (–OH) group.
Due to the large amount of free energy created when ATP is disrupted to generate adenosine diphosphate (ADP) in the phosphate-phosphate bond, this phosphorylation process is one-way.
Phosphorylation is a critical step in controlling protein function across a wide range of proteins and is strongly connected to protein activity. By causing conformational changes in the phosphorylated protein, phosphorylation regulates protein function and cell signalling.
All of these phosphorylation changes have a two-fold effect on the protein. To begin, conformational changes in the protein are used to monitor its catalytic activity. As a result, phosphorylation can either activate or deactivate a protein.
Second, phosphorylated proteins attract surrounding proteins with structurally preserved domains that recognise and connect to phosphomotifs. The amino acids that differentiate these domains are distinct.
Phosphotyrosine (pY) selectivity is demonstrated by the Src homology2 (SH2) and phosphotyrosine-binding (PTB) domains, however variations between these two kinds are unique to various phosphotyrosine species.
Phosphoserine (pS) is recognised by both the MH2 and WW domains, but phosphothreonine (phosphorylated threonine) (pT) is recognised by forkhead-associated FHA domains.
The ability of phosphoproteins to attract other proteins, in which phosphorylated signal protein is recruited downstream, is important for signal transduction.
Protein phosphorylation is one of the reversible post-translational modifications (PTM) that is controlled by kinases to phosphorylate and phosphatases to dephosphorylate substrates. The phosphorylated protein dynamics behaviour in a cell is promoted by both enzyme types.
In reality, the size of the phosphoproteome in a single cell is determined by the spatial and temporal equilibrium of kinase and phosphatase concentrations inside the cell, as well as the catalytic performance at a specific location.
Protein Kinases
Kinases are enzymes that help phosphate transfer to substrates. More than 500 kinases have been predicted from the human proteome. This group of proteins can be found in the human kinome.
Lipids, carbohydrates, nucleotides, and proteins are among the substrates for kinase action. While guanosine triphosphate is utilised in a few kinases, ATP is nearly universally used as a co-substrate in protein kinases.
ATP is ideal for the transition of α-, β– or γ- phosphate classes in nucleotidyl-, pyrophosphoryl-, and phosphoryl conversion. The ATP binding site is generally preserved, although kinase substrate selectivity varies.
Protein kinases are divided into subgroups based on their catalytic domains, which include tyrosine kinases and serine/threonine kinases. Serine / threonine kinases account for about 80% of the mammalian kinome, while phosphoproteomes pS and pT account for more than 90% of the phosphoproteome.
According to research, the relative abundance ratio of pS:pT:pY in a cell is 1800:200:1. Despite the fact that pY is not as common as pS and pT, global tyrosine phosphorylation is at the top of biomedical science’s priority list due to its link to human sickness via the dysregulation of receptor tyrosine kinases (RTKs).
The protein kinase substrate’s specificity is determined not only by the desired amino acid, but also by the consensus sequences that surround it. In certain kinases, these consensus sequences allow for the phosphorylation of a single protein as well as many phosphorylated substrates (>300).
As a result, kinases may be able to phosphorylate single or multiple amino acids in individual proteins if kinase-specific consensus sequences are available.
Control subunits in kinases operate as activating or auto-inhibiting domains with various regulatory substrates. Phosphorylation of these subunits is a common method of regulating kinase activity. Most protein kinases are dephosphorylated and inactive in their natural state. Only a small percentage of phosphorylated kinases are fundamentally inefficient or inactive because they are constitutively active.
Phosphorylation and dephosphorylation must be combined in some kinases, including Src, indicating that this proto-oncogene is tightly controlled. By regulating the spatial connection between kinases and upstream and downstream regulators, scaffolding and adaptor proteins can also influence kinase behaviour.
The activity of certain kinases can be determined by incubating immunoprecipitate with substrates of different kinases and ATP. Commercial instruments are available for this type of test, and they can accomplish colorimetric, radiometric, or fluorometric identification.
Although this type of test shows the activity of specific kinases, including kinase enrichment, it does not show the proteins that kinases alter or the influence of endogenous phosphatase activity.
Signal Transduction Cascades
Because protein phosphorylation is reversible, cells may easily adjust to intracellular or external stimuli, this type of PTM is appropriate for signal transmission. One or more proteins that sensitively represent the physical sensors by binding a ligand, cleavage, or some other response differentiate signal transduction cascades of the signal.
When kinases are phosphorylated, these receptors activate downstream, then phosphorylate and activate their cognate downstream substrates, as well as other kinases, before reaching the exact response.
Signal transduction pathways can be linear, with kinase A activating kinase B, which then activates kinase C, and so on. Kinases A trigger multiple kinases, which in turn excite more kinases, revealing signalling pathways that amplify the initial signal. With this type of communication, a single molecule can induce widespread cellular activity like proliferation, for example.
Protein Phosphotases
The pace and breadth of phosphorylation-related signalling are controlled by three pathways:
1. Separation of the stimulating ligand from the other ligands
2. Proteolysis of a substrate by a kinase
3. Phosphatase Dephosphorylation
Around 150 phosphatases of pS, pT, and pY residue types are known to be present in the human proteome. Although both phosphatase groups have the same ultimate goal of dephosphorylation, they go about it in distinct ways.
Serine/threonine phosphatases, bimetallic centres (Fe/Zn), and tyrosine control quick hydrolysis in the phosphorus group of the phosphorus atom. Phosphatases produce a thiophosphoryl covalent intermediate that permits tyrosine residues to be removed.
In living cells, hosphorylation is crucial since it is through this process that energy-rich molecules (such as ATPs) are produced. Phosphorylases and kinases, for example, catalyse the addition of a phosphoryl group (phosphate) to ADP, resulting in ATP.
Phosphorylation Types
Phosphorylation and dephosphorylation may occur in a variety of substances.
1. Glucose Phosphorylation
When glucose is phosphorylated with different other sugars during its catabolism, it is referred to as phosphorylation. D-glucose is first transformed to D-glucose-6-phosphate in glycolysis, for example. Glucose is a tiny molecule that penetrates quickly. Because glucose is so little, it gets into the cells fast.
Phosphorylation is a larger enzyme that can’t get into the tissue rapidly. Phosphorylation is also necessary for the control of blood glucose levels. The synthesis of glycogen is directly proportional to the content of glucose. Phosphorylation of glucose is also linked to heart development.
2. Protein Phosphorylation
Phosphorylation of proteins occurs when an amino acid is linked to a phosphorylate group. Even though phosphorylation occurs with threonine and tyrosine in eukaryotes and histidine in prokaryotes, serine is the most common amino acid.
When one phosphate group interacts with the hydroxyl (-OH) group of a serine, threonine, or tyrosine side chain, this is referred to as an esterification process.
Protein kinase attaches the amino acid to the phosphate group in a covalent manner. The mechanism differs significantly between prokaryotes and eukaryotes.
The best-studied kind of phosphorylation appears to be post-translational modifications (PTM), which indicate that proteins are phosphorylated after conversion from an RNA sequence. Dephosphorylation and reversal processes were performed by protein phosphatases.
Protein phosphorylation may be seen in the phosphorylation of histones, which is a good example. In eukaryotes, DNA is linked with chromatin-forming histone proteins.
Histone phosphorylation alters the DNA-protein and protein-protein interactions, affecting chromatin shape. When DNA is degraded, phosphorylation occurs to provide new locations across damaged DNA so that repair procedures can operate correctly.
In addition to its importance in DNA repair, protein phosphorylation plays an important role in metabolism and signalling cascades.
3. Oxidative Phosphorylation
Oxidative phosphorylation is the process through which a cell releases and absorbs chemical energy. In the eukaryotic cell, reactions occur within the mitochondria. Interactions in the electron transport chain and chemiosmosis are involved in oxidative phosphorylation.
Finally, the transfer of electrons from molecules and proteins into the inner membrane of mitochondria via the electron transport chain produces the energy needed to generate adenosine triphosphate (ATP) in chemiosmosis.
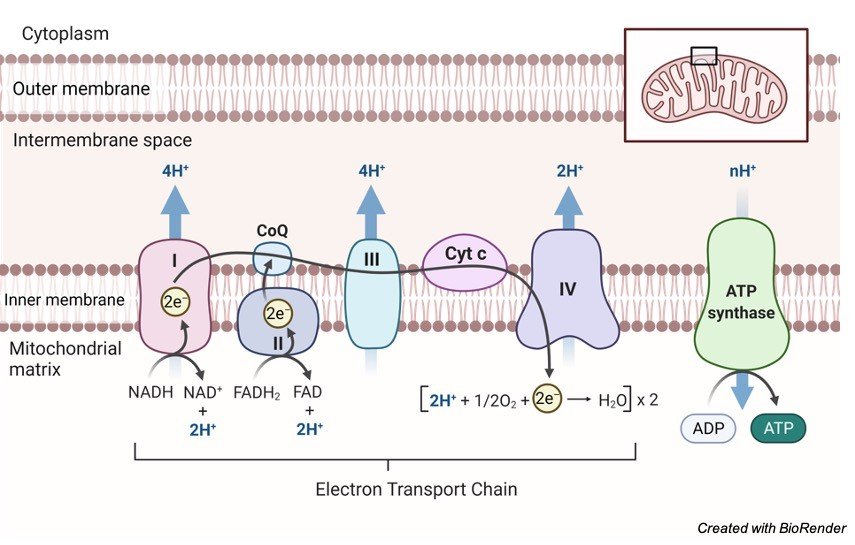
NADH and FADH2 send electrons to the electron transport chain during this procedure. As electrons go down the chain, they release energy as they go from higher to lower energy. Much of the energy used to create an electrochemical gradient is spent on transporting hydrogen ions (H+).
By interacting with H+ ions at the network’s end, electrons are transformed to oxygen and water is created. The ATP synthesiser receives ATP synthase energy from these ions.
As ATP is dephosphorylated, the phosphate group is degraded, producing energy that may be used by the cell. It’s possible that adenosine isn’t the sole base that gets phosphorylated to make AMP, ADP, and ATP. Guanosine, for example, has the potential to produce GMP, GDP, and GTP.
Detecting Phosphorylation
The phosphorylation of a molecule is detected using a mass spectrometer, electrophoresis, and antibodies. Phosphorylation sites, on the other hand, are difficult to identify and describe. Fluorescence, electrophoresis, and immunoassays are all employed in conjunction with isotope marking.
Biological Importance of Phosphorylation
Organic phosphates, which help living organisms develop tissues through anabolic chemical reactions, are important in the transfer of energy.
The importance of organic phosphates in metabolism was demonstrated and the findings were reported in Harden and Young’s study, which showed that when inorganic phosphorus was applied to the media and then converted to organic phosphate, the fermentation of glucose by cell-free yeast juice quickly improved.
Physiological Importance of Phosphorylation
Phosphoric acid is used to make the cell protoplasm. Phosphorylation is thus a crucial chemical activity for all cells. It’s also important for absorbing and metabolising a wide variety of foods.
The following is a list of its effects on several metabolic pathways:
In relation to Carbohydrates:
1. Phosphorylation aids carbohydrate absorption by the intestinal mucosa as well as glucose reabsorption by the kidneys. Hexose phosphate is produced in each of these epithelial cells. This molecule undergoes dephosphorylation, allowing hexoses to enter the circulation while leaving phosphoric acid alone.
2. Glycogen synthesis from glucose and glycogen breakdown into glucose are hypothesised to occur during phosphorylation in the liver and muscles.
3. Phosphorylation is common at all phases of chemical changes that occur after muscle contraction. Phosphorylation and dephosphorylation occur in this area in a cycle. Glycogen is broken down into lactic acid by a number of phosphorylated molecules.
In relation to Fats:
1. The absorbing epithelium produces neutral fats and phospholipids mostly during fat digestion. The enzyme phosphorylase is responsible for phosphorylation.
2. Phospholipids, particularly lecithin, are produced by the liver. The transfer of fat is an important stage. It also acts as the primary step of fatty acid oxidation. Fatty acid oxidation is a mitochondrial activity.
In relation to Proteins and Vitamins:
All phosphoproteins, including nucleoproteins, caseinogens, and others, must be produced. Phosphorylation is so expected to have a role in tissue oxidation, which is the process by which proteins, lipids, and carbohydrates are destroyed.
When it comes to the vitamin, it’s a no-brainer. Compounds like riboflavin phosphate, thiamine pyrophosphate, and others are found in certain vitamin B groups.
They are thought to be coenzymes in the oxidation and reduction phases of cell metabolism.
Regulation of Phosphorylation
The adrenal cortex is considered to be the source of the phosphorylation. Glucocorticoids increase the inhibition of phosphorylation after adrenalectomy.
Through its adrenocorticotropic hormone, the anterior pituitary may offer sophisticated phosphorylation control to the adrenal cortex.
The marasmic condition of adrenal cortex disorders may be explained in part by phosphorylation disturbance, which impairs absorption, metabolism, and body nutrition.
Phosphorylation Citations
Share
Similar Post:
-

Plants: Definition, Types, and Examples
Continue ReadingPlants Definition
As defined by botany, any eukaryotic species of the biological kingdom Plantae that is photosynthetic and has a rigid cell wall. Planta is a Latin word that means “sprout, shoot, cutting.”
Plants Characteristics
A plant is any eukaryotic organism that belongs to the Plantae biological kingdom. In the strictest sense, plants are embryophytes, that included vascular plants, liverworts, hornworts, and mosses. In certain less stringent references, green algae was considered a plant in certain less stringent references. Green algae include both unicellular and multicellular species with chloroplasts and cell walls.
Autotrophs are plants that eat just their own kind of food. Photosynthesis is how they produce their own nourishment. They can capture energy through the chloroplast’s green pigment (chlorophyll) and use carbon dioxide and water to create carbohydrates as food and oxygen as a by-product.
Plants are typically found at the top of the food chain since they are autotrophs. They’re referred to as “producers.” Other creatures, especially mammals, consume them as food. Animals, on the other hand, are heterotrophs, meaning they must devour other species to survive.
Some animals (especially herbivores) consume just plants, whereas others eat only meat or a combination of animal and plant matter. Plants do not rely on animals to develop and thrive since they are capable of producing their own nourishment.
A group of carnivorous plants (such as the Venus flytrap) that catch and devour animal prey, primarily when photosynthesis is difficult, is an exception.
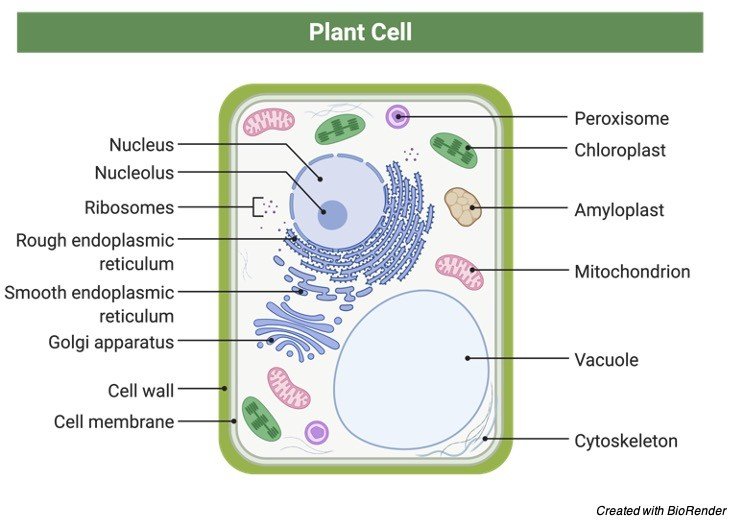
Plants are eukaryotes, or multicellular organisms. Plants, like mammals, have a membrane-bound nucleus inside their cells. The nucleus is an organelle that houses genes on chromosomes. Golgi apparatus, endoplasmic reticulum, lysosomes, peroxisomes, and plastids are further organelles suspended in the cytoplasm of a plant cell.
Plastids are found in plants. Plastids inside a eukaryotic cell indicate that it is more likely a plant than an animal. Plastids are available in a wide range of forms and sizes.
Chloroplasts are photosynthesis-related plastids that contain chlorophyll (green pigments). Apart from green, chromoplasts include pigments and are involved in pigment production and storage. Light energy is absorbed by chlorophyll systems at certain wavelengths in the electromagnetic spectrum.
Pigments also contribute to the colour of plant structures (e.g. green leaves, red flowers, yellow fruits). Non-pigmented plastids, such as amyloplasts, elaioplasts, and proteinoplasts, are known as leucoplasts. Their primary use is to store food. Sugar, such as starch, is used by plants to store food.
Inside the cell of plants, there is a huge vacuole. This cytoplasmic structure is important in turgor pressure control.
Apart from the plasma membrane, plants have stiff cell walls. A plant cell’s cell wall provides additional structural support.
Plants do not have a skeletal structure like animals, but their cell walls are largely made of cellulose material, which helps to provide structural support.
Plants have a unique cell division system in which daughter cells are separated by a cell plate (phragmoplast). Plants do not have the same mobility as mammals. They are unable to go from one area to another at their leisure. As a result, they must contend with extreme weather, such as heat. Their cell walls, which keep their bodies from drying up, are one of the ways they can endure heat.
Plants, meanwhile, continue to move, albeit in a different way. The folding of the leaflets of the plant Mimosa pudica when touched, for example, and the shutting of the leaf of the Venus flytrap while trapping prey are both examples of nastic movement.
Some plants, such as the silver birch Betula pendula, would even droop their branches and leaves at night, as if they were “sleeping.” Tropism is another type of plant movement.
Tropism, on the other hand, is more of a response to a stimulus than a movement. Plants, for example, prefer to grow towards the light source (phototropism).
Plasmodesmata are plasmodesmata found on plants. Plants contain plasmodesmata, which behave as if they were cell connections between plant cells, but animals have cell junctions that keep cells in an animal tissue.
These cytoplasmic bridges between neighbouring cells are formed by the cell wall. These “bridges” assist maintain the tonicity of plant cells by facilitating cell contact and allowing fluid circulation.
Plants are multicellular, with numerous cells organised into tissues and organs that work together to accomplish a specific purpose.
Anchorage, support, and photosynthesis are all specific functions of plant organs (e.g. roots, stems, leaves, etc.) Plants can grow indefinitely thanks to their meristematic tissues. Indeterminate, actively dividing cells make up the tissue, which gives birth to differentiated tissues including epidermis, trichomes, phellem, and vascular tissues.
Plants do not have sensory organs, yet they may detect their surroundings in a variety of ways. Despite their absence of eyes, ears, and noses, plants can “see,” “hear,” and “smell.” They appear to “feel” and respond in ways that animals do not.
Plants may not have the same nervous system as mammals, but they appear to have their own system based on how they respond to their environment.
Despite not having eyes, Arabidopsis has photoreceptors (at least 11 kinds) that let the plant perceive light. Herbivory, for instance, might cause the release of specific compounds on the damaged plant component.
Herbivores are deterred by the production of defensive compounds by plants. Tomatoes have been seen emitting volatile signals to warn adjacent plants of an imminent herbivore assault.
Plants reproduce in two ways: asexually and sexually. Plants reproduce asexually by budding, fragmentation, fission, spore development, vegetative propagation, apomixis, and other methods.
Male and female gametes merge during fertilisation, resulting in sexual reproduction. In general, the plant life cycle includes generational alternation, or the sporophyte and gametophyte stages alternating.
Plants are able to “breathe.” Carbon dioxide from the atmosphere enters the plant cell through stomata. Carbon dioxide is transformed to oxygen during photosynthesis, which the plant releases as a metabolic by-product into the environment through the stomata.
Plants may lack other biological systems, but they do generate compounds that aid in plant defence and immunological activities, as well as plant hormones that operate as signalling molecules.
Plant Body
Plant Body Embryophytes have two primary organ systems: one for the shoots and another for the roots. The shoot system comprises of body components in the plant’s top section, whereas the root system consists of body parts in the bottom region.
Plant parts such as stems, branches, leaves, flowers, and fruits may all be found in the shoot system. They are frequently seen above ground. Roots, tubers, and rhizomes are all part of the root system. They are frequently discovered underground.
Plant tissues include the following:
1. Plant tissues made up of undifferentiated and mitotically active cells are known as embryonic or meristematic tissues. Apical meristem and cambium are two examples.
2. Permanent tissues are differentiated cell-based plant tissues. Fundamental (e.g., parenchyma, collenchyma, sclerenchyma) and complex (e.g., parenchyma, collenchyma, sclerenchyma) permanent tissues can be further divided (e.g. phloem and xylem tissues)
3. Reproductive tissues are those parts of the plant that are engaged in reproduction. The sporogenous tissues are one example.
Plant cells are eukaryotic, meaning they have a well-defined nucleus. The chromosomes that carry genes are found in the nucleus. The endoplasmic reticulum, Golgi apparatus, mitochondria, lysosomes, and plastids are the other organelles except the nucleus.
Chloroplasts (containing chlorophyll, a green pigment), chromoplasts (with colours other than green), and leucoplasts (with pigments other than green) are the three types of plastids (colorless plastids). The vacuole is a huge structure inside the plant cell. It’s in charge of keeping turgor pressure in check.
The cytoplasm, where these organelles are suspended, is surrounded by the plasma membrane. The cell has a second layer, the cell wall, in addition to the plasma membrane. The cell wall, on the other hand, is not unique to embryophytes. Other species with cell walls include fungus, algae, and certain bacteria.
Primary and secondary cell walls make up the cell walls of embryophytes. Cellulose, hemicelluloses, and pectin make up a main cell wall. A thicker layer is the secondary cell wall. It contains a lot of lignin, which helps to reinforce and waterproof the wall.
One of the numerous functions of the cell wall is to aid in the resistance to osmotic pressure. Water flows into a plant cell when it is put in a hypertonic solution, causing the cell to expand. During severe osmosis, the existence of the cell wall prevents the cell from bursting.
Water diffuses out of a plant cell when it is put in a hypotonic solution, and turgor pressure is reduced, causing the cell to become flaccid. Further water loss will cause plasmolysis, which will eventually lead to cytorrhysis, or the total collapse of the cell wall.
Photosynthesis, respiration, transpiration, tropisms, nastic movements, photoperiodism, circadian rhythms, seed germination, and dormancy are all essential physiological activities that plants do.
Plant Genomics
Plants’ genomes are rather big. With roughly 94,000 genes, the wheat Triticum asestivum genome is the biggest of the plant genomes that have been sequenced.
Plant Life Cycle
Plants have two generations in their life cycle: gametophyte generation and sporophyte generation. Alternation of generations refers to the transition between diploid and haploid forms. Certain algae, such as Archaeplastida and Heterokontophyta, have this behaviour.
The sporophyte and the gametophyte are separate organisms in algae with generations that alternate. The gametophyte generation in embryophytes is one in which the phase begins with a haploid spore (n). To become a gametophyte, the spore goes through a sequence of mitotic divisions.
A haploid multicellular plant type is known as a gametophyte. There would just be one pair of chromosomes. Because the gametophyte phase is the sexual phase of the life cycle, the plant will grow sex organs that generate haploid gametes. Following the union of gametes, the gametes that participated in fertilisation would join the sporophyte generation, which is characterised by a diploid plant form.
The sporophyte is the main phase of tracheophytes (vascular plants) and is multicellular. As a result, the sporophyte is the most visible plant. The gametophyte, on the other hand, is the dominant plant in bryophytes (mosses and liverworts), and hence the predominant plant we see.
Tracheophytes’ life phases begin with a seed, which develops into a scion when conditions are favourable for development. The scion develops leaves, stalks, and branches as it grows. It grows into an adult plant that produces blooms in the end.
Sex cells are found in the flowers, such as sperm cells in pollen grains and ova in the ovary’s ovules. The seed contains a zygote formed by the fusion of the sex cells. Plants that are monoecious have both sex cells, whereas dioecious plants only have one kind of sex cell.
Plants can reproduce asexually as well. They achieve this by avoiding the use of gametes. Budding, fragmentation, fission, spore generation, vegetative propagation, and apomixis are all examples of asexual reproduction.
The ageing process of plants is referred to as plant senescence. During leaf senescence, for example, the yellowing of leaves happens as a result of chlorophyll breakdown, leaving only the carotenoids. However, certain plants, such as deciduous trees, may continue to produce new leaves.
Plant Ecology
Plants do not need to hunt or eat animals for nourishment since they are capable of photosynthesis (with the exception of carnivorous plants). They can create their own food by combining light energy, atmospheric carbon dioxide, and water molecules.
Nonetheless, the waste that animals exhale during breathing is one source of carbon dioxide. They give out oxygen as a waste product of photosynthesis in exchange. Animals, like other aerobic creatures, require oxygen to survive.
Other essential nutrients are obtained by plants from dissolved minerals in the soil. They take them in via their roots.
Calcium, magnesium, nitrogen, phosphorus, potassium, and sulphur are some of the macronutrients they get from the soil. Plants absorb boron, chloride, copper, iron, manganese, and molybdenum as micronutrients.
As a result, the breakdown of dead plant parts, or the entire plant, results in the return of important minerals and chemicals to the Earth.
They are frequently put at the top of a food chain due to their feeling of independence. In an ecosystem, they are the primary producers. As a result, plant extinction can have a significant influence on an ecosystem.
The Red List of Threatened Species of the International Union for Conservation of Nature (IUCN), a system for monitoring the conservation status of species across the globe, used a method of categorising species based on extinction risk. As a result, species can be classified as “data deficient,” “near-threatened,” “vulnerable,” “endangered,” “critically endangered,” “regionally extinct,” “extinct in the wild,” and “extinct.”
In 2016, the International Union for Conservation of Nature (IUCN) listed 2,493 plants as critically endangered and 3,654 species as endangered.
Plants develop symbiotic relationships with other species. Here are several examples:
1. Mutualism – for example, plants provide nectar for honeybees, while honeybees aid in the dispersion of pollen grains.
2. Predation – for example, carnivorous plants that eat insects and other tiny animals
3. Competition – for example, plants that compete for available space and nutrients with other plants for habitat.
4. Commensalism – for example, plant fruits that adhere to animal hair for free transportation.
5. Parasitism – for example, parasitic plants that obtain nutrients from their hosts, such as Cuscuta (dodder), which attaches to an acacia tree and develops haustoria that absorb nutrients.
The Census of Marine Life projected in 2011 that there might be about 8.7 million eukaryote species on Earth, including about 298,000 plant species. There have previously been 215, 644 described and catalogued.
Plant Evolution
Organelles such as plastids and mitochondria, according to the endosymbiotic hypothesis, reflect once free-living prokaryotes. The chloroplasts appear to be connected to cyanobacteria, which are prokaryotic microorganisms.
The structural similarity between cyanobacteria and chloroplasts provides the foundation. Furthermore, they share the same photosynthetic pigments as the genome and a single circular DNA molecule.
Endosymbiotic processes, it appears, were responsible for the emergence of the earliest photosynthetic eukaryotes one billion years ago. The embryophytes are thought to have evolved from Charophyta (a kind of green algae).
Many similarities exist between charophytes and embryophytes, such as the development of phragmoplasts during mitosis.
The following is a short chronology of embryophyte evolution:
Phanerozoic aeon » Paleozoic era » Ordovician period: The first embyophytes (land plants) arose during the Ordovician epoch (485 million years ago to 440 million years ago).
Phanerozoic eon » Paleozoic era » Devonian period: Primitive plants, trees, and shrub-like forests dominated the earth throughout the Devonian epoch (415 million to 360 million years ago), providing new habitats for terrestrial creatures. Elkinsia, an early seed fern, developed seeds, especially in the late Devonian era.
Phanerozoic eon » Mesozoic era: This period lasted 252 million to 66 million years. Flowering plants first occur in the Triassic period (about 200 million years ago).
Phanerozoic eon » Cenozoic era: The “new life” epoch, which stretches from 66 million years ago to the present day, is the most recent geological epoch. The grasses first developed during this time period, about 40 million years ago. To withstand the low CO2 and dry conditions of the tropics, these plants and many other plant species developed a novel metabolic process.
Plant Taxonomy
Because they all include chloroplasts and a cell wall, green algae, fungus, and embryophytes are included in the original definition of plants. Algae and fungus, on the other hand, were finally assigned to their separate kingdoms.
Plants (i.e. Plantae sensu strictissimo) are multicellular organisms having cellulose-containing cell walls and chloroplasts for photosynthesis in the strictest meaning. The kingdom Plantae is made up of embryophytes, which include vascular plants, liverworts, mosses, and other fossil plants with similar characteristics.
Embryophytes and green algae are included in Plantae sensu stricto (“plants in a limited sense”) (Chlorophyta and Charophyta). This concept of plants is still commonly used today. They belong to the Viridiplantae (or Chlorobionta) group, which is also known as the green plants.
The following are the divisions of the kingdom Plantae sensu stricto: Chlorophyta, Charophyta, Marchantiophyta (liverworts), Anthocerotophyta (hornworts), Bryophyta (mosses), Lycopodiophyta (club mosses), Pteridophyta (ferns, whisk ferns, and horsetails), Cycadophyta (cycads), Ginkgophyta (ginkgo), Pinophyta (flowering plants).
Significance of Plants
Plants are necessary for the survival of many creatures since they are the food chain’s producers. They are capable of storing starch. They’re also a valuable source of minerals and chemicals.
Plants provide homes for a variety of creatures, e.g. insects and arboreal organisms. They are also the most important source of oxygen for aerobic creatures.
Medicinal qualities can be found in some plants. Plantain (Plantago major) leaves for decreasing inflammation and discomfort, and burdock (Arctium minus) roots and leaves for eczema and damaged skin are just a few of the many therapeutic plants.
Essential oils, pigments, resins, tannins, alkaloids, amber, waxes, cosmetics, plastics, rubber, varnish, lubricants, inks, and a variety of other goods are all made from plants.
Buildings, musical instruments, boats, and furniture are all made from plant-based wood. It’s also utilised in the production of paper.
Plant Research
Botany is the field of science that examines plants (or plant biology). A botanist is a specialist in this subject. Some of the disciplines of study comprise morphology, cytology, histology, physiology, ecology, evolution, taxonomy, and pathology. Sub-disciplines emerged as a result of the diversity of plant groupings, including:
1. Paleobotany is the science of studying fossil plants.
2. Algology is the study of algae.
3. Mycology is the study of fungi.
4. Bryology is the study of mosses, liverworts, and hornworts.
5. Pteridology is the science of ferns.
6. Pollen grains and spores are studied in palynology.
Applied botany is concerned with the commercial and economic applications of plants. Agriculture (for example, agronomy, horticulture, and plant breeding), forestry (for example, dendrology, wood technology), pharmaceutical botany, and landscape architecture are all included.
Plants Citations
- Allelopathic Plants: Models for Studying Plant-Interkingdom Interactions. Trends Plant Sci . 2020 Feb;25(2):176-185.
- Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev Cell . 2020 Dec 7;55(5):529-543.
- Unraveling salt stress signaling in plants. J Integr Plant Biol . 2018 Sep;60(9):796-804.
Share
Similar Post:
-

Top 10 Cheapest Online Criminal Justice Programs
Continue ReadingWhat are Criminal Justice Programs?
Criminal Justice Programs are dynamic field where professionals can find exciting positions in criminology, national security, and other professions. While some professions only require a high school diploma, a college education improves essential professional skills and lays the foundation for professional development.
From leadership opportunities to learning new skills, studying criminal justice can help those interested in criminal justice or are already working in it to get a rewarding job in the field.
Online Criminal Justice Programs
For those wary of investing in four years of study, there are a number of affordable criminal justice programs available online. With the flexibility and affordability of the remote format, students are increasingly choosing to complete their criminal justice degrees online.
While cost will undoubtedly keep students motivated when choosing their school, it is important that frugal students when it comes to tuition look beyond the cheapest online criminal justice programs and look for programs that are worth the investment of time and money.
What Are the Cheapest Criminal Justice Programs?
We compiled list of top 10 universities offering cheapest Online Criminal Justice Programs*
1. University of South Florida Tampa, FL
2. California State University, Stanislaus Turlock, CA
3. Florida International University Miami, FL
4. Florida State University Tallahassee, FL
5. University of Central Florida Orlando, FL
6. California State University, San Bernardino San Bernardino, CA
7. California State University, Sacramento Sacramento, CA
8. Florida Gulf Coast University Fort Myers, FL
9. Rutgers, State University from New Jersey New Brunswick, NJ
10. Missouri State University Springfield, MO
* The result is a list that goes beyond the cheapest online criminal justice programs and takes into account the total economic value each program offers to online criminal justice students and data could vary.
Universities Offering Cheapest Online Criminal Justice Programs
The University of South Florida’s main campus is in Tampa, and it serves over 50,000 students each year. A number of academic programmes are available, including an online Bachelor of Applied Science in Criminal Justice Programs. Distance learners can choose to follow an entirely online curriculum or combine the two by taking a few classes in person.
The 120-credit programme includes topics such as theories of criminal conduct and a mentored analysis of the criminal justice system. The University of South Florida requires first-year candidates to submit their ACT or SAT results. The university employs the Canvas platform to supervise its students from afar.
More than 60 academic programmes are available at California State University, Stanislaus. A combined Bachelor of Arts programme in criminal justice is available in the Bachelor Catalog, and it prepares students for professions as court administrators, lawn inspectors, and crime scene investigators.
Courses such as criminal causes, juvenile justice, and criminal procedure are included in the curriculum. In addition, students learn about police ethics and civil accountability in the context of race and ethnicity. The programme concludes in a highlight with a focus on empirical research after the students complete an internship.
Stan State’s Online Criminal Justice Programs require applicants to have a minimum of 60 transferred credits and a 2.0 GPA to be eligible for graduation. Candidates for this fast track must meet on campus one evening each week in a hybrid setting.
Miami-based Since 2000, Florida International University has provided accessible and career-focused distant learning programmes. An online crime science degree, for example, teaches students how to mix computer science and forensic techniques with classic criminology and criminal justice topics.
Courses on behavioral and social science statistics, crime and terrorism prevention, and national security are all required. Students also discuss cybersecurity topics such as the reasons of cybercrime and how ethical hacking might be used as a deterrent. A police officer training programme that allows students to acquire college credits while pursuing their dream of becoming a cop.
Autumn, spring, and summer start dates are available at FIU. Applicants must submit their ACT or SAT scores to be considered for this programme. Students who study online pay tuition dependent on where they live.
Tallahassee-based Florida State University is a comprehensive public university with more than 200 academic departments. Many remote choices are available in your catalogue, including: An online criminology degree that prepares students for careers as fraud investigators, probation officers, and corporate security specialists by teaching them investigative and analytical abilities.
Criminal Justice Administration and Victimology are two required courses. Students also learn about the social realities of black men, media representations of crime and victimhood, and violence in the United States. Authorities from the state or the federal government. ACT/SAT scores, a personal statement, and an up-to-date CV are needed of applicants for Online Criminal Justice Programs. There is no requirement for distance students to come to campus.
With more than 90 distance learning academic programmes, the University of Central Florida has long been a leader in distant learning. Students can acquire a bachelor’s degree in criminal justice or a bachelor’s degree in arts. Graduates are qualified for jobs as FBI agents, crime scene investigators, and correctional officers.
Courses in US crime and prosecution / jurisprudence are required. BA candidates must show competency in a foreign language equivalent to one year of college education, as well as an understanding of the importance of humane policies and procedures.
Online Criminal Justice Programs employs a thorough evaluation procedure that considers the candidate’s grade point average, ACT/SAT scores, and work experience. Canvas at UCF allows for distance learning.
Every year, over 20,000 students enrol at California State University, San Bernardino, with many of them earning their credentials online through Blackboard. Justice in Criminal Matters This programme allows students to work full-time and graduate in two years.
Compulsory studies in the Bachelor’s degree in criminal justice curriculum include criminal law and theories / institutions of the penal system. Students get practical skills in applied research and statistical analysis, as well as the opportunity to explore personal interests and aspirations through six electives, which include community corrections. cross-border organised crime, and forensic profiles
A minimum of 60 transferable credits (meeting all state-mandated general educational requirements) and a GPA of 2.7 are required of candidates. CSUSB offers a range of incentives, including institutional scholarships based on performance.
The College of Continuing Studies at California State University, Sacramento provides a number of flexible certificates and degrees. Students in the school’s fully online Bachelor of Science in Criminal Justice programme will attend asynchronous courses on Canvas and gain the management and communication skills needed to work in law enforcement, probation and probation systems, and courts.
Candidates must pass core courses in general investigation tactics, criminal law, and sentence execution fundamentals. You’ll also learn about research methods and how to use them in a final seminar that looks at current difficulties in the criminal justice profession.
Online Criminal Justice Programs needs an associate degree or a minimum of 60 transferable courses with a 2.0 GPA as a prerequisite for graduation. The fall and spring shooting dates are maintained by the state of Sacramento.
Students can earn a bachelor’s degree in criminal justice by completing all upper division (class 60) course requirements at Gulf Coast University in Fort Myers, Florida, through Canvas College. It’s a web-based programme.
Online Criminal Justice Programs cover criminal behaviour theory, juvenile justice, and the criminal process under the law. Students also look at leadership and management tactics in local, state, and federal governments. Students must complete an internship to get a Bachelor of Criminal Justice degree.
FGCU’s average freshman has a 3.87 GPA, and candidates must provide ACT or SAT scores. The university provides a variety of financial aid opportunities, including merit scholarships, tuition waivers for overseas students, and military and international personnel awards.
Rutgers is a well-known public research university with around 71,000 students enrolled each year.
A bachelor’s degree in Online Criminal Justice Programs can be completed entirely online or as part of a hybrid distance learning program.
The undergraduate degree in criminal justice covers topics such as police and society, law enforcement, crime and juvenile justice skills, and data analysis as a policy tool.
Freshmen require ACT / SAT scores and a personal essay on a specific question. Online students use Blackboard to access asynchronous classes.
Graduates can go on to earn a master’s degree or serve as US Marshals or Police Officers. Juvenile Justice and Institutional/Community Corrections are mandatory courses. Unlike many other criminal justice programmes, this one permits students to specialise in criminal investigation, terrorism and national security, or environmental law to supplement their education.
Applicants must present ACT or SAT scores that meet the minimum scale standards for their grade point average. Candidates who do not meet these requirements might take advantage of an alternative admissions process that includes an individual screening and summer enrollment.
Share
Similar Post: