Author: Admin
-
Cell Viability Assay: Neutral Red Uptake Assay...
Continue ReadingAbout Neutral Red Cell Viability Assay
The NRU cytotoxicity assay or Neutral red uptake assay procedure is a cell survival or cell viability chemosensitivity assay based on the ability of live cells to incorporate and bind neutral red (NR), a supravital dye.
Principle of Neutral Red Cell Viability Assay
NR is a weak cationic dye that rapidly enters into cells via cell plasma membranes by non-ionic diffusion and accumulates intracellularly in lysosomes.
Cell surface alterations or the sensitive lysosomal membrane lead to lysosomal fragility and other modifications that gradually become irreversible.
These changes brought about by the action of any toxic compound result in a decreased uptake and binding of NR.
It is thus possible to distinguish between dead cell, viable cells, damaged cells, which is the basis of the NRU cytotoxicity assay or Neutral red uptake assay.
Healthy and viable mammalian cells, when maintained in culture conditions, continuously proliferate and divide over time.
Any chemical compound that will interfere with this process and result in a reduction of the growth rate due to toxic nature of compound as reflected by cell number or cell death.
Cytotoxicity is expressed as a concentration dependent reduction of the uptake of the NR after chemical exposure thus providing a sensitive, integrated signal of both cell integrity and growth inhibition.

Created with BioRender
Neutral Red Cell Viability Assay Requirement
Sterile:
1. Secondary cell line
2. Growth medium with 10% FCS; Growth medium with 5% FCS
3. Trypsin (0.25%) + EDTA, (1 mM) in PBS
4. Multiwell plates (96 well), Reservoir for multichannel pipette.
5. Pipettor tips, in an autoclavable tip box
6. Universal containers or tubes
7. Cytotoxic drug, 70% IPA
8. Neutral red stock solution (3.3 mg/ml in water)
9. Neutral red medium (1:100 dilution of neutral red stock in medium)
10. Neutral red desorbing medium(50% ethanol, 1% glacial acetic acid)
Non-sterile:
11. Plastic box (clear polystyrene, to hold plates)
12. Multichannel pipettor, neuber’s chamber
13. Dimethyl sulfoxide (DMSO)
14. ELISA plate reader
Neutral Red Cell Viability Assay Procedure
Cells are plated in 96 well plate at a concentration of 1×104 cells / well.
The medium used is RPMI-1640 medium supplemented with10% FBS (Fetal Bovine Serum).
The plate is incubated for 24 hr at 37 C in CO2 incubator with 5% CO2.
The dilutions of the drugs are prepared in RPMI-1640 medium supplemented with 5% FBS.
After 24 hr of seeding, medium is removed and cells are treated with different concentrations of drug.
Treatment is done in 96 well plates and is same as given for MTT assay.
150 μl of drug containing medium is added per well and incubated for 72 hr at 37oC in CO2 incubator.
Control wells are treated with drug free medium. DMSO control wells are treated with medium containing DSMO (as calculated by the dilutions used for the assay)
After the incubation period of 72 hr, the medium is removed.
150 μl of Neutral red medium is added to each well and the plate is incubated at 37C in CO2 incubator.
After the incubation period of 3 hr, medium is removed and cells are washed with 1X PBS and 100 μl of neutral red desorbing solution is added.
Record optical density (O.D.) at 540/570 nm using ELISA plate reader.
Cell viability is calculated as a ratio of control and plotted against log concentration of drug to calculate the IC50.
Analysis of Neutral Red Cell Viability Assay
Plot a graph of the absorbance (y-axis), considering cells without drug as 100% against the concentration of drug (x-axis).
Calculate the LC50 as the drug concentration that is required to reduce the absorbance to half that of the control.
Neutral Red Cell Viability Assay Citations:
Share
Similar Post:
-

Phases of Growth, Growth Rate, and Growth...
Continue ReadingWhat is Cell Growth
Growth is an irreversible, exponential progressive process which results in the increase in the biomass of an organism both quantitatively and qualitatively over a period of time thereby making an organism fit for survival in every better possible way.
Growth is a net result of accumulation of small changes initiating at cellular level and contributing in large scale over a period of time.
Plants show a remarkable feature of infinite cell divisions at the specialized region of meristems where the stem cells are present waiting for an internal stimulator to initiate growth.
When a sapling starts its growth period the meristems were present all over the plant diving continuously and increasing exponentially the number of cells in plant.
Later, the region of meristem gets restricted to specific parts such as the Apex, Laterals, and Intercalary.
Phases of Growth
With appropriate nutrients and favorable environmental condition, a plant undergoes desirable change is gradual steps. The changes initiates from cells forms 3 phases of growth.
They are:
A. Cell Division
B. Cell Enlargement
C. Cell Differentiation
A. Cell Division
The initial process of plant growth is the division of cells by the process of DNA replication and cell growth.
Cell cycle mitosis becomes very essential as it is associated with somatic cell proliferation.
The division takes place in meristems where the stem cell proliferates to increase the length and girth of a plant.
Mitosis involves the 4 stages of cell division producing 2 cells by single division. The four stages are Prophase, Metaphase, Telophase and Anaphase followed by Cytokinesis and G0 Phase (i.e.) Quiescent phase.
The mitosis cycle is dependent on the Cyclin Dependent Kinase (CDK) that regulates the synthesis, replication and cell division.
I. Prophase: Initiation of Karyokinesis
1. DNA synthesis is complete at this stage
2. Condensation of chromosome
3. Chromosome has 2 chromatids connected by a centromere
4. Duplicated centrosome migrates to the poles of the nuclear membrane
5. Centrosome releases microtubules forming aster. The microtubules reach centromere of each chromosome by forming spindle fibers
6. The disintegration of nuclear membrane initiates at the end of the prophase
7. The cell organelles start to disappear
II. Metaphase: Chromosomal Alignment
1. Nuclear membrane disintegration initiates the stage of metaphase
2. Chromosome becomes distinct with sister chromatids held together by centromere
3. The centromere surface has kinetochores. Kinetochores are binding region for the spindle fibers and are disc shaped
4. Spindle fibers align the chromosomes at the equator of the cell called metaphase plate
5. The chromosome is now arranged equatorially in the cytoplasm attached by spindle fibers
III. Anaphase: Sister Cell is Formed
1. The third stage of mitosis carries one half of chromosome to respective poles
2. Centromere drags the chromatids to opposite poles
IV. Telophase: Separation of Daughter Cells
1. Chromatids groups at the poles of the cell
2. Nuclear membrane is generated around the chromatids
3. Chromatids decondense to become chromatin reticulum
4. Reappearance of lost cell organelles – Golgi apparatus, Endoplasmic reticulum, etc.,
V. Cytokinesis
1. The process of division of one cell into two daughter cells after mitosis is cytokinesis
2. A cell plate is formed in between nucleus
3. The cell plate formation begins with small furrow in between 2 daughter nuclei
4. The furrow extends further to the lateral sides and the cells separate from each other
5. Cell plate is formed by the Golgi Body vesicles namely phragmoplast.
6. The orientation of nuclei is controlled by actin, myosin II and regulatory proteins forming a contractile ring
7. Cell division is accomplished by expansion in size of daughter cells
8. The new plasma membrane is formed by the fusion of intracellular vesicles
The cell division or the cell formation phase takes place at the region of meristems in the plant.
B. Cell Enlargement
Enlargement of cell is the maturation phase where cell size increases to acquire nutrient for the coordinated growth.
The enlargement takes place horizontally where the inner cell wall has high solute content increasing the osmotic pressure for the water to enter.
The entry of water is stored in the vacuolated cells, which increase in size.
High water quantity makes the cell diluted and turgid.
The cell wall now becomes thin. Golgi apparatus has a clear role in the formation of the pressure inside the cell.
This function is also regulated by the hormonal influences of the body along with cytoskeleton.
C. Cell Differentiation
In this phase the cell completely matures and retains stem cell for dedifferentiation.
The phase provides a clear distinction between permanent tissue and meristematic tissues.
Growth Curve
The sigmoid curve representing the rate of growth is the growth curve. The overall growth of the plant is simply represented in the curve.
Four phases of curve are plotted. Namely: Lag Phase, Log Phase, Diminishing Phase and Stationary Phase.

1. Lag Phase: the initial growth period is referred as lag phase. In this phase each cell starts to divide continuously and make itself easily available to uptake of nutrients and increase cell mass. The phase involves gradual increase in cell growth.
2. Log Phase: the rapid cell growth period is the log phase. Under Favorable environmental condition the cell growth increases exponentially in large scale by the multiplication of cell division. Simultaneous nutrient input and maturation takes place in this stage. However, the cell division exceeds the maturation
3. Diminishing Phase: the cells start maturation providing a higher yield of cellular metabolites. The growth or new cell formation is confined to certain region of meristems which divides but to keep up with overall plant growth. Reduces the rate of formation of new cells
4. Stationary Phase: A final stage of plant growth where the meristematic regions constantly produce new cells and old cells are removed. This constant maintenance of cell cycle is the Stationary Phase.
Phases of Growth Citations
- Linear growth retardation in relation to the three phases of growth. Eur J Clin Nutr . 1994 Feb;48 Suppl 1:S25-43; discussion S43-4.
- The diagnostic performance of dental maturity for identification of the circumpubertal growth phases: a meta-analysis. Prog Orthod . 2013 May 23;14:8.
- The influence of growth patterns on sexual size monomorphism in lemurs. J Evol Biol . 2015 Sep;28(9):1670-81.
Share
Similar Post:
-

Breast Cancer : Types of Treatment Options...
Continue Reading"Main challenge with the surgery is the recurrence of the tumor from the cells which are left during surgery there should not be a single tumor cells left during surgery"
Therapies for breast cancer
Surgery: Surgical removal of visible cancer cells and tissues by experts to avoid spread of the tumor to other parts of the body. Amount of tissues removed depends upon the size of the tumor.
There are many types of surgeries needle biopsy, lumpectomy, mastectomy, modified mastectomy are common types of surgeries in breast cancer.
During needle biopsy minute amount of cells or tissue is removed with the help of needle under the guidance of imaging techniques.
Needle biopsy is done for the diagnostic purpose. In lumpectomy a small lump of the tumor tissue is removed for the diagnosis purpose.

"Usually surgery follows chemotherapy or adjuvant chemotherapies to further eradicate the remaining breast cancer cells and prevent the recurrence of the disease"
In mastectomy a large portion of the breast removed along with normal surrounding tissue while in modified mastectomy tumor tissue, surrounding normal tissue along with armpit lymph node are removed are removed.
Main challenge with the surgery is the recurrence of the tumor from the cells which are left during surgery there should not be a single tumor cells left during surgery.
Surgery is also associated with inflammatory complications. Usually surgery follows chemotherapy or adjuvant chemotherapies to further eradicate the remaining cells and prevent the recurrence of the disease.
Sometime radiation therapy is recommended after mastectomy to reduce the recurrence of the tumor.
"When breast cancer cells exposed to high energy radiation such as X-rays cancer cells are not able to repair the DNA damage"
Radiotherapy: It involves the administration of ionizing radiation, X-ray or gamma rays to the tumor site.
Normal cells suspend cell division when their DNA get damage by halting their DNA replication does not proceed until get repaired when the DNA damage is irreparable cells undergoes to programmed cell death.
Cancer cells are genetically unstable cells which has lost the power repairing the DNA damage for their own benefits.
When cancer cells exposed to high energy radiation such as X-rays cancer cells are not able to repair the DNA damage induced by high energy radiations and finally get died while normal cells repairing mechanism is intact and are able to repair the damage induce by these radiations.
So these radiations specifically targets cancer cells.

Gibhardt CS et. al, 2015, showed that challenging cells with ≥1 Gy X-rays or with UV-A laser micro-irradiation causes a rapid rise of H2O2 in the nucleus and in the cytosol which result in the production and glutathione-buffering, is sufficient for triggering a signaling cascade that involves an elevation of cytosolic Ca2+ and eventually an activation of hIK channels.
A course of radiation therapy is preceded by a simulation session in which low-energy beam are used to produce radiograghic images that indicate the exact beam location.
Radiation therapy is usually delivered in fractionated doses such as 180 to 300 cGy per day, five times a week for a total course of 5-8 weeks.
Success of radiotherapy depends in the difference in the radio sensitivity between the tumor and normal tissue.
Radiation therapy either used individually or in combination with chemotherapy.
Although radiation therapy is effective against many types of cancers this therapy is also associated with side effects, Fatigue and serious skin problems (dryness, itching, blistering, or peeling), GI toxicity, oropharyngeal mucositis& xerostomia, myelosuppression.
There is also possibility of developing a secondary cancer after radiation therapy.
"Radiation therapy either used individually or in combination with chemotherapy to treat breast cancer"
Chemotherapy: Chemotherapy is a treatment method for cancer with one or more chemically synthesized anti-cancer drugs which mainly targets rapidly dividing cancer cells and normal cells (epithelial cells of mouth, intestine lining).
Chemotherapy either given intravenously or orally in cycles.
Usually chemotherapy given after surgery or mastectomy to kill cancer cells which are left during surgery to reduce recurrence of tumor.

Alkylating agents, antimetabolites, antitumor antibiotic, plant alkaloids, hormonal agent, immunotherapy are the important agents of chemotherapy.
The most common chemo drugs used for early breast cancer include the anthracyclines such as doxorubicin and the taxanes ( paclitaxel and docetaxel).
These may be used in combination with certain other drugs, like fluorouracil (5-FU), cyclophosphamide, and carboplatin while for advance breast cancer docetaxel, paclitaxel, cisplatin, carboplatin, liposomal doxorubicin, mitoxantrone, vinorelbine etc.
Chemotherapy is associated with loss of appetite, hair loss, mouth sores, nausea and vomiting, low blood cell counts increases the chance of getting infection, irregular menstrual cycle and fatigue.
E.G, Myelosuppression, nausea &vomiting, Stomatitis and alopecia.
"Chemotherapy is associated with loss of appetite, hair loss, mouth sores, nausea and vomiting, low blood cell counts increases the chance of getting infection, irregular menstrual cycle and fatigue"
Hormone therapy or endocrine therapy: A gold standard therapy for ER positive breast cancers.
The main purpose of hormone therapy is to reduce the level of steroids (estrogens, androgens and progesterone) in the body or blocking the actions.
But hormone therapy does not work in ER negative breast cancer. Aromatase inhibitors, selective estrogen receptor modulators (SERMs) and selective estrogen receptor down regulators (SERDs) mainly use in hormone therapy.
"Hormone therapy or endocrine therapy is a gold standard therapy for ER positive breast cancers"
In alternate chemotherapy fallopian tube, ovary and adrenal glands surgically removed to reduce the hormone level in the body.
Tiredness, weight gain, hot flush, menopausal symtoms, digestive problems, headache, hair thinning, sweating, breast tenderness, memory problems and mood swing are associated side effects of the endocrine therapies.

Biological therapy or Targeted therapy: Targeted therapy is the use of drugs which specifically target the cancer by interfering the molecular targets of the cancer cells which have important role in growth and progression of cancer cells.
Most of the targeted drugs are cytostatic which block the cell proliferation.
Molecular targets are decided by comparing the molecular profiles of the normal and cancer cells and those proteins which are over expressed in cancer cells are selected as molecular target such as HER2/neu over expressed in ER negative breast cancer cases.
Trastuzumab, a monoclonal antibody against HER2/neu protein used to target the over expressed HER2/neu, effective therapy for the ER negative HER2/neu over expressing breast cancer cases.
"Most of the targeted drugs are cytostatic which block the breast cancer cell proliferation"
Another approach is to analyse the protein for the mutation which only found in cancer cells and specifically target the mutated proteins.
Target therapy includes hormone therapies, signal trasducer inhibitors (gefitinib), gene expression modulators, apoptosis inducer, angiogenesis inhibitors (bevacizumab), cancer vaccine, gene therapy and immunotherapies.
Toremifene, Trastuzumab, fulvestrant, exemestane, lapatinib , letrozole, pertuzumab, ado-trastuzumab emtansine, palbociclib, Everolimus, tamoxifen, anastrozole are the targeted drugs approved for the treatment of breast cancer.
Skin problems, high blood pressure, problem with blood clotting and wound healing are the associated side effects of the targeted therapies.

Bone Directed therapy: When breast cancer cells metastases to bone serious complications arises. Bisphosphonates (pamidronate, zoledronic acid) and denosumab are mainly used in such conditions. Biphosphonates mainly increases the bone strength and reduces bone thinning.
Osteonecrosis is serious side effect with the use of biphosphonates.
Denosumab another drugs for the bone directed therapy it is more effective than biphosphonates. Low blood calcium and phosphates are the side effects associated with Denosumab.
Share
-

Electrophoresis: an Overview, How to Run Gel...
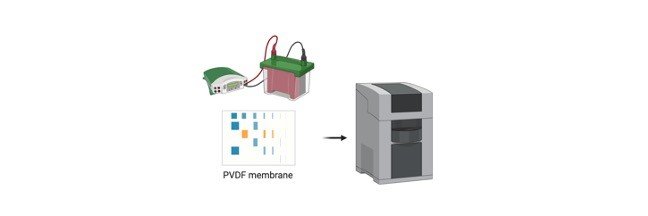
Continue ReadingGel Electrophoresis Objective
To perform SDS-PAGE Gel Electrophoresis for separation of proteins.
About Gel Electrophoresis
Almost most of the analytical electrophoresis of proteins is carried out in polyacrylamide gels under conditions (generally SDS-PAGE) that ensure dissociation of the proteins into their individual polypeptide subunits.
Most common anionic detergent SDS (Sodium Dodecyl Sulfate) is used in combination with a reducing agent such as 2-mercaptoethanol or dithiothreitol (DTT) followed by heating to dissociate or break ionic and disulphide bonds of the proteins before they are loaded on the gel for Gel Electrophoresis.
The denatured proteins or polypeptide binds SDS and become negatively charged.
Because the amount of SDS bound is proportional to the molecular weight of the polypeptide or protein and is independent of its sequence, SDS-polypeptide complexes migrate through polyacrylamide gels in accordance with the size of polypeptide.
Using various markers of known molecular weight, it is therefore possible to estimate the molecular weight of the polypeptide chain(s).
Hence, SDS-PAGE Gel Electrophoresis is the most widely used method for qualitatively analyzing any protein mixtures.
The method is based on the separation of proteins according to their size and then locating by binding them to a dye.
Gel Electrophoresis is particularly used for monitoring protein purity and to determine their relative mass.
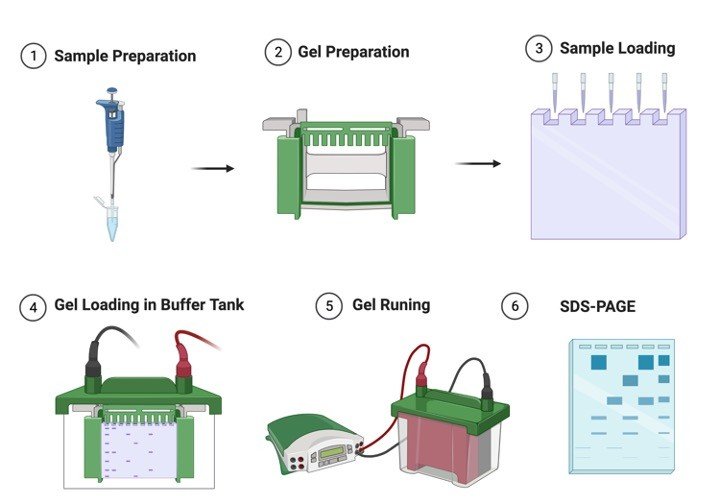
Adopted from BioRender
In, most cases SDS-PAGE Gel Electrophoresis is carried out in a discontinuous buffer system in which buffer in the reservoirs is of a various pH gradient and ionic strength from the buffer used to cast the polyacrylamide gel.
The Sodium Dodecyl Sulfate (SDS)-polypeptide complexes in the protein sample that is applied to the gel are swept along by a moving fence created when an electric current is passed between the electrodes.
After moving through a stacking gel of high porosity, the protein complexes are stacked on the surface of the resolving gel.
The ability of discontinuous buffer systems is to concentrate all of the complexes into a very small volume greatly increases the resolution of Sodium Dodecyl Sulfate (SDS)-polyacrylamide gels.
Mechanism of Gel Polymerization in Electrophoresis
The sample and the stacking gel contain Tris-Cl (pH 6.8), and upper top and lower buffer tank contain Tris-glycine (pH 8.3), and the resolving gel contains Tris-Cl (pH 8.8).
All components of the system contain 0.1% SDS.
The gels are composed of chains of polymerized acrylamide that are cross-linked by a bifunctional agent such as N,N’ -methylene bisacrylamide. The effective range of separation of Sodium Dodecyl Sulfate (SDS)-polyacrylamide gels (SDS-PAGE ) depends on the concentration of polyacrylamide used to prepare the gel and on the amount of cross-linking.
Polymerization of acrylamide in absence of cross-linking agents generates viscous solutions that are of no practical use.
Cross-liks formed by bisacrylamide add rigidity and tensile strength to the gel and form pores through which the SDS-polypeptide complexes must pass.
The size of these pores decreases with the increase of bisacrylamide:acrylamide ratio, reaching a minimum when the ratio is approximately 1:20.
Most gels are cast with molar ratios of bisacrylamide:acrylamide of 1:29.
The sieving properties of the gel are evaluated by the size of the pore which is determined by the absolute concentrations of acrylamide and bisacrylamide.
The following table shows the linear range of separation obtained with gel cast with concentrations of acrylamide that range from 5% to 15%:
Acrylamide Concentration and Protein Size in Electrophoresis
Acrylamide concentration (%) Linear range of separation (kD) 15 12-43 10 16-68 7.5 36-94 5.0 57-212 Component of Gel Electrophoresis
Separating / resolving gel:
The lower (separating, running or resolving) gel is prepared using 5-15% acrylamide which is more than that used in the stacking gel (the amount of acrylamide used depends upon the molecular weight of the macromolecule under separation).
Hence the pores are numerous and of a smaller diameter imparting the molecular sieving property to this gel.
It is in this gel that the macromolecules subsequently separate.
The separating gel constitutes about two-thirds of the length of the gel plates.
The buffer used in the running gel is Tris.Cl at pH 8.8.
Stacking gel:
The stacking gel is layered on top of the separating gel after it has polymerized completely.
It is prepared using 2-5% of acrylamide and is consequently highly porous and devoid of any molecular sieving action.
The stacking gel constitutes one-third of the length of the gel plates in which the comb is placed in order to form wells for sample loading.
The buffer used in preparation of stacking gel is Tris.Cl. at pH 6.8.
Gel Electrophoresis Process
Glycine in the upper buffer reservoir exists in two forms; as a zwitterion which does not have a net charge, and as glycinate ion which is negatively charged.
When the power is switched on, chloride, protein and glycinate anions begin to migrate towards the anode.
Upon entering the stacking gel, the glycinate ions encounter a condition of low pH (pH of stacking gel buffer is about 2 pH units lower than that of the upper reservoir) which shifts the pH towards formation of zwitter ion.
Since zwitter ions are devoid of charge, they are immobile.
This immobility of glycine zwitterions to migrate into stacking gel coupled with high mobility of the chloride ions creates high localized voltage gradient between the leading chloride ion and the trailing glycinate ions.
Since protiens have their mobility intermediate between the trailing and the leading ions, the proteins carry the current in this region and migrate rapidly in this high local electric field.
However, they cannot overtake the chloride ions since the strong local fields exist only between the chloride and the glycinate ions.
As a consequence the proteins migrate quickly until they reach the chloride rich region and then drastically slow down.
This two speed movement of the proteins results in a piling up of protein sample in a tight sharp disc between the glycinate and the chloride ions.
It is in this form that the protein enters the resolving gel.
The small pore size of the gel retards the protein band for a while which allows the glycinate ions to catch up.
As soon as the glycinate ions enter the resolving gel they encounter a higher pH range and thus resume their full charge upon diasappearance of the localized high voltage gradient.
Now the separation of the proteins takes place according to their molecular weight.
Note: The buffer in the upper and the lower reservoir is Tris-glycine at pH 8.3, whereas the buffer used for preparing the stacking gel is Tris-Cl at pH 6.8.
Gel Electrophoresis Requirements
1. Acrylamide and N,N’ -methylene bisacrylamide: Prepare a stock solution containing 29% (w/v) acrylamide and 1% (w/v) bisacrylamide in deionized warm, water (to assist the dissolution of bisacrylamide).
Note: Acrylamide and bisacrylamide are slowly converted to acrylic and bisacrylic acid upon storage.
The reaction is catalyzed by light and alkali.
Hence always check that the pH of the solution is 7.0 or less, and store it in dark bottles at room temperature.
The fresh solutions should be prepared every few months.
Caution: Both are neurotoxins.
Polyacrylamide is considered to be non-toxic but care is to be taken while handling it as it might contain some amount of unpolymerized material.
2. Sodium dodecyl sulphate (SDS): Prepare a 10% (w/v) stock solution in Deionized water and store at room temperature.
3. Tris buffers for the preparation of resolving and stacking gels: Prepare 1.5 M Tris Cl. (pH 8.8) for resolving gel and 1.0 M Tris.Cl (pH 6.8) for stacking gel.
4. TEMED (N,N,N’N’-tetramethylenediamine): TEMED accelerates the polymerization of acrylamide and bisacrylamide by catalyzing the formation of free redicals from ammonium persulfate.
5. Ammonium persulfate (APS): It provides free radicals for polymerization of acrylamide and bisacrylamide.
Ammonium persulfate decomposes slowly and fresh solution is to be prepared weekly.
Prepare a 10% stock solution in Deionized water and store at 4C.
6. Tris-glycine electrophoresis buffer: 25 mM Tris base, 250 mM glycine and 0.1% SDS.
Adjust pH 8.3.
Prepare a 5X stock by dissolving 15.1 g of Tris base and 94 g of glycine in 900 ml water.
Adjust pH 8.3.
Add 50 ml of 10% (w/v) stock solution of SDS.
Make up the volume to 1000 ml with water.
7. 2X SDS gel-loading buffer: 100 mM Tris Cl (pH 6.8), 10% β-mercaptoethanol, 4% SDS, 0.2% bromophenol blue, 20% glycerol.
Gel Electrophoresis Procedure
1. Clean the whole Gel Electrophoresis apparatus with methanol so as to ensure them to be clean and detergent free.
Assemble the glass plates.
Clamp the assembly to the gel casting apparatus.
Ensure the Gel Electrophoresis assembly is leak proof by filling methanol between the plates where the gel is to be casted.
2. Determine the volume of the gel mold.
3. Prepare the resolving gel as shown below:
For preparing 15 ml, 12% gel
Solution components Volume in ml Water 4.9 30% acrylamide mix 6.0 1.5M Tris.Cl (pH 8.8) 3.8 10% SDS 0.15 10% APS 0.15 TEMED .006 Add APS and TEMED in the last because polymerization will begin as soon as TEMED has been added.
4. Pour 13 ml of acrylamide solution in the gap between the glass plates (leave sufficient space for the stacking gel- the length of the teeth of the comb plus 1 cm).
Carefully overlay the gel with isobutanol to prevent oxygen from diffusing into the gel (oxygen inhibits polymerization).
5. After polymerization is complete (around 30-40 min), pour off the isobutanol and wash the top of the gel several times with water so as to remove the traces of unpolymerized material.
Remove the remaining water using a filter paper towel.
6. Prepare the stacking gel as follows:
Solution components Volume in ml Water 5.5 30% acrylamide mix 1.3 1.0M Tris.Cl (pH 6.8) 1.0 10% SDS 0.08 10% APS 0.08 TEMED .008 8. Pour the stacking gel solution directly on to the surface of the polymerized resolving gel.
Immediately insert a clean Teflon comb (cleaned with water and dried with ethanol).
Avoid trapping air bubbles.
Add more stacking gel solution from the sides of the comb to fill the spaces completely.
9. After polymerization is complete (30-40 min), remove the Teflon comb, clean the wells with water to remove any unpolymerized material.
Mount the gel in the electrophoretic apparatus.
For ease mark the wells.
Add tris-glycine electrophoretic buffer to the top and the bottom reservoir.
First add the buffer in the upper reservoir and check for leak.
10. Load upto 20 ul of each sample in a pre-determined order into the bottom of the wells with a Hamilton microliter syringe.
Load the samples in an order so that the gel can be cut in two exact replicas after run so as to use one part for staining and other for blotting.
11. Attach the electrophoretic apparatus to electric power supply.
Positive electrode should be connected to the lower reservoir.
Apply voltage of 8V/cm to the gel.
When the dye front has moved into the resolving gel increase the voltage to 15V/cm and run the gel until the dye reaches the bottom of the gel.
12. Turn off the power supply. Remove the gel plates from the apparatus.
Carefully remove the gel with the help of spacers.
Cut it into two and place one part in a reservoir to stain with coomassie brilliant blue so as to visualize the protein bands, and use the other part for western blotting.
Store the part to be used for western blotting in transfer buffer (39 mM glycine, 48 mM Tris base, 0.037% SDS and 20% methanol, pH 8.3.
Make up the volume with water) at 4C.
Gel Staining
Polypeptides separated by SDS-PAGE Gel Electrophoresis can be simultaneously fixed with methanol:glacial acetic acid and stained with coomassie brilliant blue R250.
The gel is immersed for several hours in a concentrated methanol and acetic acid solution of the dye and the excess dye is then allowed to diffuse from the gel during a prolonged period of destaining.
1. Dissolve 0.25 g of coomassie brilliant blue R250 (COBBR-250) in 90 ml of methanol: H2O (1:1 v/v) and 10ml of glacial acetic acid.
Filter the solution through a whatmann No. 1 filter to remove any particulate matter.
2. Immerse the gel in atleast 5 volumes of staining solution and place on a slowly rocking platform for a minimum of 4 h at room temperature.
3. After that, remove the stain and save it for future use.
Destain the gel by soaking it in methanol/acetic acid solution (step 1) without the dye on a slowly rocking platform for 4-8 h, changing the destaining solution three or 4 times.
4. The more thoroughly the gel is destained, the smaller the amount of protein that can be detected by staining with COBBR250.
5. After destaining the gels can be stored indefinitely in water containing 20% glycerol in a sealed plastic bag.
Gel Electrophoresis Citations:
Share
Similar Post:
-

Thawing of Frozen Cell Lines: Cell Revival...
Continue ReadingAbout Thawing of Frozen Cell
Cells that are cryopreserved can be used again whenever required.
The procedure of bringing frozen cells to room temperature from a metabolically inactive state to an active state without causing much damage is called as thawing.
Principle of Thawing or Cell Revival
When cryopreserved cells are needed for study, they should be thawed rapidly and plated at high density to optimize recovery.
The ampoule should be thawed as rapidly as possible, to minimize intracellular ice crystal growth during the warming process.
This can be done in warm water, in a bucket or water bath.
The cell suspension should be diluted slowly after thawing as rapid dilution reduces viability.
Some mammalian cells (often suspension-growing cells) are more sensitive to cryoprotectants, particularly DMSO, and must be centrifuged after thawing but still need to be diluted slowly in medium first.
Thawing Requirements
Sterile:
1. Culture flask
2. Centrifuge tube (if centrifugation is required)
3. Growth medium
4. Pipettes, 1 ml, 10 ml
Non-sterile:
5. Protective gloves and face mask
6. Sterile water at 37°C, 10 cm deep in a clean, alcohol-swabbed bucket with lid
7. 70% IPA, cotton swabs.
Thawing Procedure
1. Bring the cryovial out of freezer in ice. Thaw.
2. Incubate in water bath at 37o C for 1-2 minutes.
3. Double-check the label to confirm the identity of the cells; then swab the vial thoroughly with 70% alcohol, and open it in a laminar-flow hood.
4. Transfer the contents of the vial to a 2ml microfuge tube and seal with parafilm.
5. Centrifuge at 1300 rpm for 10 minutes at room temperature.
6. Resuspend the pellet in 2 ml medium.
7. Recentrifuge at 1300 rpm for 10 minutes at room temperature to remove traces of DMSO. Pellet should be resuspended in 1 ml of medium.
8. Transfer the suspension to 25 cm2 flask. Add medium slowly to the cell suspension (5 ml). For cells in suspension culture also require centrifugation to remove the cryoprotectant.
9. Incubate cells in a humidified incubator with 5% CO2, at 37oC.
10. Check after 24 h.
Post Thawing Observations
For attached monolayer cells, confirm attachment and try to estimate percentage survival based on photographs of cells at the expected density (cells/cm2 ) with full survival.
For cells growing in suspension culture, check appearance (clear cytoplasm, lack of granularity and dilute to regular seeding concentration.
This can be made more precise if the cells are counted and an estimate of viability is made, in which case the cells can be diluted to the regular seeding concentration of viable cells.
Thawing Precaution
Dilution should be slow in case of DMSO because sudden dilutions can cause severe osmotic change and reduce the viability.
On removal from storage, extreme caution must be exercised to prevent explosion of the cryo vial because of sudden expansion of the trapped nitrogen.
To retain maximum viability during cryopreservation, cells must be cooled at a constant slow rate, -1 to -5 C/min.
To minimize contamination risk, all components should be pretested.
Resuspending cells for freezing in precooled freeze medium, 0-4 C, may improve their survival prior to freezing.
When this approach is used, it is important to maintain the cells at a constant temperature during all subsequent handling by placing ampoules in ice until they are frozen.
After thawing cells it is necessary to slowly dilute the cryoprotectant to prevent osmotic shock.
If the cryovial is stored in the liquid N2 it may expand and on thawing it may crack.
Thawing Citations:
Share
Similar Post:
-

Chick Embryo Fibroblasts Cells: Growth and Maintenance
Continue ReadingFundamentals of Chick Embryo Fibroblasts
This in vitro system enables to study various stages in embryonic development of chick.
It has the advantage of providing easy access to the embryo and extra-embryonic membranes for the observation of morphogenesis and growth as well as for application of different agents under study and inoculation of viruses in specific extra embryonic membranes.
The technique does not require any sophisticated equipments, media or sera and could be used not only for research purposes but also for teaching of embryology in schools, colleges and universities.
The present model may serve as an in-vitro model suitable for the fields of developmental biology, toxicology, teratology, virology and several other aspects of biomedical research.
Materials Required for Chick Embryo Fibroblasts Culture
Freshly fertilized hen’s eggs
Unfertilized hen’s eggs
Transparent glass bowl (surface diameter 8.5 cm, bottom diameter 4.5 cm and depth 4.5 cm)
Glass petri dish (9.5 cm diameter)
70% alcohol, scalpel, incubator
Procedure for Chick Embryo Fibroblasts Culture
Wash freshly fertilized hen’s eggs with water, allow to air dry and incubate at 37.5 C with a relative humidity of 70-80% for 24 h before culturing.
Allow incubated eggs to cool for 25-30 mins, wipe with 70% alcohol under laminar flow to minimize the chances of contamination from shell surface.
Keep the eggs in the horizontal position to assure that the embryo is properly positioned.
Thin albumin from unfertilized eggs was poured into a sterile bowl.
This albumin acted as a shock absorber, provided a cushion for the culture and also limited desiccation.
Crack the fertilized, incubated egg from above with the help of a scalpel at about 3-3.5 cm from the narrow end and gently release the contents of the egg over the albumin cushion in the bowl.
Transferring of the egg contents without damage to embryo and yolk and upright position of the embryo is necessary for better survival of the embryo.
Cover the bowl with a glass petridish and incubate cultures at 37.5 C and 80% humidity.
Observe the cultures after different duration of time to obtain the embryos of various Hamburger Hamilton stages.
Embryo culture can be continued for 17-19 days.
This system has been developed and used to demonstrate the glucose induced malformations in developing chick embryos.
Chick Embryo Fibroblasts Culture Citations:
Share
Similar Research Position:
-
Plant Growth: Definition, Types, Examples I Research...
Continue ReadingWhat is Plant Growth?
Plant Growth – An irreversible, progressive increase of an organism’s mass over a period of time governed by the synchronous enlargement of basic unit of life – cell.
Every change starts from the smallest part of our body – our cell. Life started from it and gradually progressed to the present-day beings.
The change of one small amino acid which determined the survival whole living organisms on the earth.
The change was supported by an element of GROWTH. Growth is predetermined in the genetic material and are well regulated by complex array of organ system and environmental interaction in higher organisms.
Though plants evolved alongside the animals with more similar metabolic pathways, physiological functions, structural integrity etc.,
The evolution of two kingdom had a set distinct survival strategy which make them unique and well distinguished from each other.
Characteristics of Plant Growth
1. Plants have an open form of growth. These multicellular organism exhibits unlimited growth.
2. New set of features arises frequently to replace the old ones thereby increasing size, girth and survival of the plant.
3. The unlimited growth is supported by the meristems – growing regions of a plant which produces stem cells for unstopping cell proliferation.
4. As growth proceeds, the length of the plant and the girth of the trunk corresponds accordingly to support the whole plant.
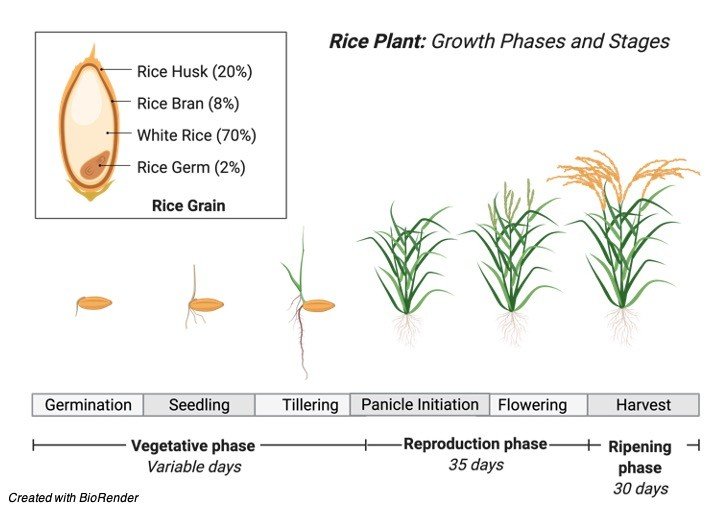
5. The plant growth can be plotted in graph against time – this forms the growth curve.
6. Cells are the basic unit of growth that takes place in 3 phases: Cell division phase, cell enlargement phase and differentiation phase.
7. Differentiation, dedifferentiation and redifferentiation are 3 main characteristic feature of cell
8. Dedifferentiation is main character of plant growth. Differentiation is a process where a cell attains a specific role and becomes a mature cell and does not divide. But plants have the process of dedifferentiation where the mature cell gain to differentiate again for cell proliferation
9. Plant growth depends upon various external factors, namely: Light, Temperature, gravity, water and touch.
10. Growth has a regulator which is fixed in the genetic material
11. The plant growth is plastic and are completely determined by the external cues.
Type of Plant Growth
Plant growth can be categorized into many types, such as:
I. Primary and secondary growth:
Primary growth is cell division of apex of root and stem and secondary growth increases the girth of the tree.
II. Limited and Unlimited growth:
When a region of plant stops growing after particular set of cell division. the growth expressed here is limited growth.
For Example: Flower, leaf, fruit.
The root system and the shoots depend only on unlimited supply of cells through cell division this forms the region to be termed with Unlimited Growth.
III. Vegetative and Reproductive growth:
Cell division and production of stem, leaf, branches without flower can be termed as vegetative Growth. Reproductive growth is a type where the plant produces the flowering part the temporary reproductive part for plant.
Plant Growth Curve
The rate of growth is determined by plotting growth against time. The curved obtained is the Sigmoid curve. The sigmoid curve represents 4 phases.
1. Lag Phase: is the initial phase where the cell proliferation starts at a slow and steady phase of growth
2. Log Phase: exponential phase where the cell proliferation takes place rapidly
3. Diminishing Phase: the rate of growth again reduces
4. Stationary Phase: a steady state of growth over time takes place
Phases of Plant Growth
Cell, division, Cell Enlargement and Cell Differentiation are the 3 phases of Plant growth.
I. Cell division: is the process where the stem cell divides into two where one half retains the ability to proliferate later (i.e.) retaining the ability of stem cell. The others half continue to proliferate.
II. Cell enlargement: The divided cell now elongates horizontally by stretching and becomes rigid. The Cell Division and Cell Enlargement are increasing the size of the cells.
III. Cell Differentiation: 3rd stage is the mature stage where the cell loses its capacity to divide further. This phase is irreversible. But the plant cells have the special ability to dedifferentiate cells for future cell division when needed.
The process of dedifferentiated cell to produce new cells through cell division is redifferentiation.
Plant Growth Hormones
Apart from all the factors said above, plant Hormones also plays a very important role in regulating growth. Hormones Such as:
1. Auxins
2. Gibberellins
3. Cytokinins
Functions of Growth hormones are:
1. They aid in cell division
2. Growth promoters for fruiting and flowerings,
3. Enlargement of cells
4. Germination of seed and formation of root.
Not only hormones certain inhibitors are present to inhibit restrictions at certain sites.
The inhibitors are:
1. Ethylene
2. Abscisic acid. Which induces seed dormancy and senescence of the organism.
Meristem
Meristems are regions of stem cell which shows unstoppable growth over the lifetime of a plant.
The main mechanism of cell proliferation is that a group of cells get differentiated from their stem cells by cell division.
One half of the cell retains the capacity of stem cell and other half gets differentiated. The differentiated cells divide continuously till their threshold and then becomes a matured cell.
In young plants all the region were stem cells, later when the plants mature the regions of cell division becomes restricted slowly and are found in localized area of an adult plant.
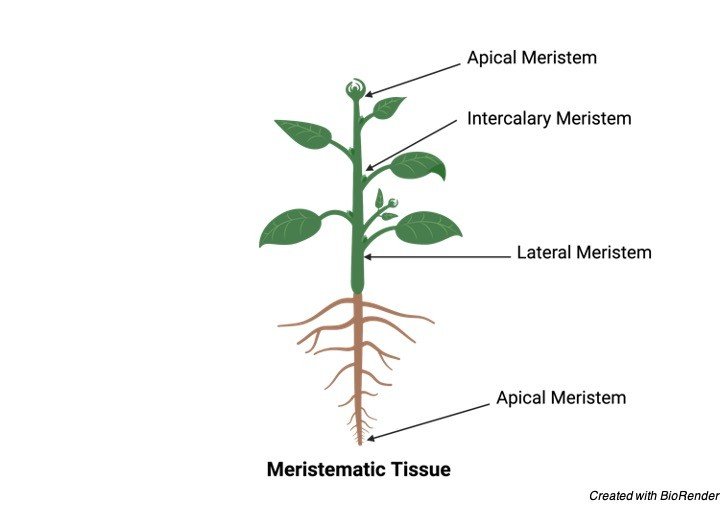
Types of Meristems
Meristem is present in both shoot and root have similar functions. Different parts of meristems are:
1. Apical Meristem: present in the tips of root and shoot. This meristem is only responsible for primary growth.
2. Lateral Meristem: Present laterally to the plants are responsible for the girth of the tree Example: Vascular Cambium, Cork Cambium
3. Intercalary Meristem: present in between mature cells
Further, the meristems can also be classified as DETERMINATE and IN – DETERMINATE. Determinates are cells whose fate is already determined who lives for short period of time like Bud, leaves etc., Indeterminates are the cell who does not lose his identity throughout their life.
Plant Growth Citations
- Radial plant growth. Curr Biol . 2017 Sep 11;27(17):R878-R882.
- Cytokinin signaling in plant development. Development . 2018 Feb 27;145(4):dev149344.
- The Phloem as a Mediator of Plant Growth Plasticity. Curr Biol . 2019 Mar 4;29(5):R173-R181.
- Molecular Aspects of Plant Growth Promotion and Protection by Bacillus subtilis. Mol Plant Microbe Interact . 2021 Jan;34(1):15-25.
- Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int J Mol Sci . 2018 Mar 22;19(4):952.
- Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol . 2012 Apr;28(4):1327-50.
Share
Similar Post:
-
Comet Assay Full Protocol to Assess DNA...
Continue ReadingAbout Comet Assay
Several man-made chemicals find their way into the environment and pose health risk to human population.
These chemicals have been found to interact with the vital tissue macromolecules regulating the cellular functions leading to long lasting health disorders.
Acute and chronic exposure to environmental chemicals such as pesticide, metals, polycyclic aromatic hydrocarbons (PAHs), radiation and others have been shown to produce marked toxicity at the target sites.
Some of these chemicals affect the DNA, which is the carrier of inherited information and any gross change in its structure potentiates serious biological changes.
Hence there is a need to test the chemicals for their genotoxic potential before being released into the environment.
The conventional methods for evaluating genetic damage include chromosomal aberration, micronucleus assay, sister chromatid exchanges.
However, these are time consuming, resource intensive and require proliferating cell population.
Hence, newer and more sensitive test systems have now been introduced for assessing the genotoxicity of chemicals.
Fundamental Of Comet Assay
The comet assay and microgel electrophoresis (MGE) were first introduced by Ostling and Johanson in 1984.
This was a neutral assay in which the lysis and electrophoresis were done under neutral conditions.
The image obtained looks like a “comet” with a distinct head, comprising of intact DNA and a tail, consisting of damaged or broken pieces of DNA hence the name “Comet” Assay was given.
The extent of DNA liberated from the head of the comet was the function of the dose of damage.
The above neutral assay was modified by two groups, Singh and co-workers (1988) and Olive et al (1989). Singh et al used microgels, for electrophoresis under highly alkaline conditions (pH>13).
This enables the DNA super coils to get relaxed and unwind, which are then pulled out during application of electric-current which made possible detection of single strand breaks in DNA and alkali labile sites expressed as frank single strand breaks in individual cells.
This method was developed to measure low levels of strand breaks with high sensitivity.
Olive and co-workers conducted the electrophoresis under neutral or mild alkaline condition (pH 12.3) to detect single stranded breaks.
This method is optimized to detect a subpopulation of cells with varying sensitivity to drug or radiation.
Importance of Comet Assay
The single cell gel electrophoresis or comet assay is one such state-of-the-art technique for the measurement of DNA damage and repair in vitro in any eukaryotic cell and some prokaryotic cells.
This technique is rapid, non-invasive, sensitive, visual and inexpensive as compared to the conventional techniques and is a powerful tool to study factors modifying mutagenicity and carcinogenicity.
In addition, it combines the simplicity of biochemical techniques for detecting DNA single strand breaks (strand breaks and incomplete excision repair sites), alkali-labile sites and cross-linking with the single cell approach typical of cytogenetic assays.
Comet assay measures, double strand breaks (DBSs), single strand breaks (SSBs), alkali labile sites, oxidative DNA base damage, DNA-DNA/ DNA-protein/ DNA-Drug cross linking and DNA repair.
Comet Assay Principle
The assay works upon the principle that strand breakage of the super coiled duplex DNA leads to the reduction of the size of the large molecule and these strands can be stretched out by electrophoresis.
Comets form as the broken ends of the negatively charged DNA molecule become free to migrate in the electric field towards the anode.
Two principles in the formation of the comet are:
1. DNA migration is a function of both size and the number of broken ends of the DNA
2. Tail length increases with damage initially and then reaches a maximum
Advantage of Comet Assay
1. It is a non-invasive technique.
2. It requires <10,000 cells and collection of data at the level of the individual cell, allowing for more robust types of statistical analyses.
3. Counting of 50-100 cells per individual/ treatment group through computerized image analysis software gives a complete analysis.
4. Any eukaryotic cell population (nasal and buccal mucosal cells, epithelial cells, male germ cells, fine needle biopsy) is amenable to analysis.
5. Duration of this experiment is few hours only whereas conventional cytogenetic assays require few days.
6. Single strand breaks (SSBs) and alkali labile lesions (capable of being transformed into SSBs under alkaline conditions) in the DNA of individual cells can be assessed.
Materials Required for Comet Assay
Frosted slides
Coverslips
Lymphocyte Separation Medium
Hydrogen peroxide (H2O2): 25 μM and 50 μM
Phosphate Buffer Saline (PBS) (i.e. 137mM NaCl, 2.7mM KCl, 10mM Na2HPO4.2H2O and 2 mM KH2PO4, pH 7.4).
Dulbecco’s modified Eagle Medium (DMEM)
Fetal Bovine Serum (FBS)
0.4% Trypan blue dye
Normal melting point agarose (NMPA)
Low melting point agarose (LMPA)
Propidium iodide
Lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Trizma base).
Neutralising Buffer (Add 0.4 M Tris to ~800 ml distilled water. Adjust the pH to 7.5 using conc. HCl to 1000 ml with distilled water).
Electrophoresis buffer (Add 30 ml 10N NaOH and 5 ml 200 mM EDTA to 1000 ml with distilled water, mix well. Ensure pH > 13 before use).
IX-51 inverted research microscope
Horizontal gel electrophoresis units
Sorvall Centrifuge
Comet Assay Procedure
Isolation of lymphocytes:
Blood was collected in microfuge tubes containing heparin (10 IU heparin per ml of blood)
Blood was diluted with 1X PBS in the ratio of 1:3 (Blood: PBS) and layered on lymphocyte separation medium (LSM) (1 ml of LSM per 3 ml of diluted blood)
Tubes were centrifuged at 400 g for 15 minutes at 15oC.
Lymphocyte layer was collected and diluted with 1X PBS in the ratio of 1:1.
Again it was centrifuged at 400 g for 10 mins at 15oC to get the lymphocyte pellet.
Pellet was resuspended in 1X PBS.
Estimation of cell viability using trypan blue exclusion assay. The standard trypan blue assay was carried out on isolated lymphocytes as mentioned in earlier protocol for cell counting.
Chemical treatment of cells:
An aliquot of cells were treated with two different concentrations of H2O2 (25 μM and 50 μM) for 15 minutes and the other aliquot of untreated cells were used as negative control.
A layer of 1% normal melting point agarose (NMPA) was prepared on frosted-end slides and slides were kept overnight at 37oC.
After chemical treatment, cells (1 x 106 cells/ml) were mixed with 1ml of 2% low melting point agarose (LMPA).
The suspension of 200 μl LMPA and cells was layered on to the precoated slides and covered with cover slips.
The layer was allowed to solidify at 4oC for 15 mins.
After solidification of the layer, cover slips were removed.
Slides were immersed in cold lysis solution (pH 10) and kept at 4C for 3 hours.
Slides were washed gently with alkaline electrophoresis buffer (pH 13) for 15 mins, twice.
This helps in allowing denaturation of DNA.
Subsequently, slides were transferred to an electrophoresis tank with fresh alkaline electrophoresis buffer and electrophoresis was performed at field strength of 20 V/ 250 mA for 25 min at 4C.
Slides were neutralized in chilled distilled water for 5 min and stained with 5 μg/ml propidium iodide for 20 mins.
DNA damage Analysis in Comet Assay
For visualization of DNA damage, observations are made with PI stained DNA using a 10X/20X objective on a fluorescent microscope.
Any image analysis system may be suitable for the quantification of SCGE data.
CASP, freely downloadable software, is used to assess the quantitative extent of DNA damage in the cells by measuring the length of DNA migration and the percentage of migrated DNA.
The software calculates the tails moment and olive tail moment.
Generally, 50 to 100 randomly selected cells are analyzed per sample.
The softwares are designed to differentiate comet head from tail and to measure a variety of parameters including tail length; % of total fluorescence in head and tail; and ‘tail moment’, calculated in different ways but essentially representing the product of tail length and relative tail intensity.
Percent DNA in tail is linearly related to DNA break frequency up to about 80% in tail, and this defines the useful range of the assay.
Tail length tends to increase rapidly with dose at low levels of damage, but soon reaches its maximum.
It is therefore the most sensitive parameter at near-background levels of damage.
Tail moment is an attempt to combine the information of tail length and tail intensity, but suffers from lack of linearity.
DNA damage Analysis with CASP Software in Comet Assay
The CASP software can be downloaded from http://www.casplab.com
The software CASP generates the frame to limit the comet area, the line to limit the tail length, the circle to identify the comet head and the cross to identify the head centre.
The small rectangle at the bottom of each picture is generated by CASP as background reference.
Comet Assay Parameters
1. % Head DNA = (Head optical intensity/ (Head optical intensity + Tail optical intensity)) X 100
2. % Tail DNA = 100- % Head DNA.
3. Tail length = Tail length is the distance of DNA migration from the body of the nuclear core and it is used to evaluate the extent of DNA damage.
4. Tail length = Tail extent (Tail from centre) + Head extent / 2
5. Olive tail moment = Tail moment is defined as the product of the tail length and the fraction of total DNA in the tail.
Tail moment incorporates a measure of both the smallest detectable size of migrating DNA (reflected in the comet tail length) and the number of relaxed/ broken pieces (represented by the intensity of DNA in the tail).
6. Olive Tail Moment = (mean Tail- mean Head) X % Tail DNA/ 100 7. Extent Tail Moment = Tail length X % Tail DNA / 100
Comet Assay Citations:
Share
Similar Post:
-

Haemophilia: Cause, Diagnosis, Symptoms, and Treatment
Continue ReadingWhat is Haemophilia?
Haemophilia is one kind of inherited genetic disorder. This is the condition where body does not have the capability to clot the blood cells during a case of an injury or any other accidents.
This leads to the higher risk in people due to its blood accumulation in the joints or at the central nervous system.
The individuals who are not suffering so much and has only mild symptoms after an injury or an accident does not cause any serious issues.
Where as bleeding in joints results in permanent damage to the bone cells or cartilage, and bleeding in brain can cause severe headache, seizures, unconsciousness and some times it may also lead to mortality of an individual.
Types of Haemophilia
Haemophilia is generally classified into two types based on the clotting factors such as follows;
Haemophilia A occurs due to a condition where there is a production or synthesis of low amount of clotting factor VIII.
Haemophilia B is caused due to the low production of clotting factor IX.
This is considered as an inherited disorder because they are passed down from their parents where the parent carrying X chromosome with defects passes the defected X chromosome to an offspring.
It is rare that the person is affected by his self without carrying inherited gene due to the result of mutation or any other defects in the chromosome or this may also happen when the self-antibodies are reacting against the body’s own clotting factors.
The other type which is said to be haemophilia C is due to the lower levels of clotting factor X and the other type known as Para haemophilia, which is due to the minimal amounts of clotting factor V.
The haemophilia which are due to acquired conditions causes cancers, autoimmune disorders etc.
To find the ability of blood to clot is the only source of diagnosis for this disorder.
Characteristics of Haemophilia
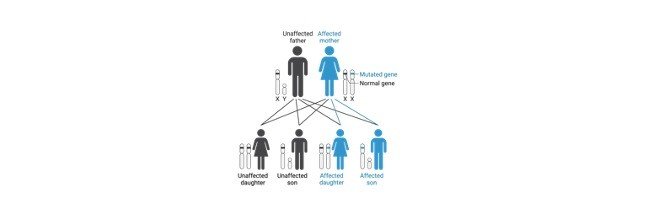
Most of the sex-linked genes are present on the X chromosome because for a simple reason that X chromosomes are larger than Y chromosomes.
Haemophilia is also an X-linked disorder, it is a type of bleeding disease which is due to the absence of clotting factors in the blood.
People who are affected with this condition have a long day bleeding or oozing of blood from the place of injury or surgery or even when the tooth is picked off.
When haemophilia undergoes a serious condition, it leads to continuous bleeding even when we met with small minor injuries, these may also result in the bleeding of joints and muscles, brain or other internal organs.
This above condition happens often in haemophilia A which is also known as classic haemophilia or factor VIII deficiency and haemophilia B condition is referred to as factor IX deficiency or Christmas disease.
These two types are almost similar in showing their symptoms and both are inherited through X- linked inherited recessive pattern.
The genes responsible for this condition are located on the X chromosome which is one of the types of allosome.
As males have only one X chromosome passing of one defective allele or mutation in one of the alleles in X chromosome can cause this condition easily, where as the females have two X chromosomes in their allosomes; So it is necessary for a female individual that both the chromosomes to get mutated or inherited to get this condition.
If only one of the chromosomes has got mutated then the respective female is considered as carrier female.
This is the reason why males are affected more than the females. It is also because the fathers do not pass the X-linked traits to their son is also to be considered.
The below graph shows how the haemophilia is being inherited.

How to Prevent Haemophilia?
We know that since it is an inherited disorder there is no chances of preventing this condition, but it can be diagnosed before the child has been given birth by the process of amniocentesis.
Where the parents are led to under a counselling to understand the risks of having a baby with this disorder and it is left to their decision whether to brought up a child carefully or terminate it.
It is the better option to consult a physician if the parents are grandparents have the condition of haemophilia. So they can come to know the results earlier.
According to research it is said that if 50 percentage if chance where the son will have haemophilia and the other 50 percentage chance is that his daughter will be carrier.
Symptoms of Haemophilia
Usually, the symptoms are based on levels of clotting factors that is present in our blood, which are responsible for clotting the blood after bleeding due to an injury or an accident.
These factors are identified during any surgical operations or while overcoming an injury with bleeding.
Symptoms of spontaneous bleeding include large and deep bruises joint pain along with swelling, unexplained bleeding and bruises, sometimes there may also a blood in urine or stools.
Nose bleeds often without having an appropriate reason, excessive bleeding and pain in the gums of the teeth and bleeding may occurs often after vaccinations.
Haemophilia Citations
Share
Similar Post:
-
Genetic Disorders: Definition, Development, and Examples
Continue ReadingWhat are Genetic Disorders
Genetic disorder is a condition where there is a mutation in a genetic material of an organism which leads to certain abnormalities.
We know that each cell in our body contains its one molecule of genetic material, which is known as DNA.
It encodes the cell to perform its particular function by giving instructions to the cell.
If there is any defect in the genetic material it leads to mutation or any other infections which leads to genetic disorders.
Cause of Genetic Disorders
Genetic disorders occurs when there is change in particular segment of a DNA or change or loss of a particular part of DNA or a whole chromosome, which is present within the body cells.
Almost all cells in our body contains an elongated strand of DNA. Each DNA is made of nucleosides and phosphate groups which encodes the cells to work in an appropriate manner so that the functioning of body remains stable.
DNA’s are placed in a chromosome, where each chromosome contains the small segment of DNA which is called as Genes.
It provides instructions to the body. Human body consists of about 23 pair of chromosomes where each parent sends their copy of one pair to their offspring’s, when such genes are passed to a next generation, a defect or mutation in a particular gene causes genetic disorder.
If the defect is passed from the past generations, it is said to be inherited. Only few people have this condition of getting inherited disorders.
Genetic disorders are classified into various types depending upon the defect in a chromosome or in a genome.
Before knowing about the disease-causing factors of gene it is important to about human genome.
Human Genome
All the gene and DNA which is required to build a human is referred to as human genome. Human Genome Project (HGP) is one of the global research projects which is used to map the human genome.
This project helps in sequencing the genes and to know about their different function.
HGP found that there are about 20,000 to 25,000 genes in the human genome. It also found the genomes and their appropriate functions which helped us to find the disease-causing factor of the genes depending upon the mutation in the bases of the DNA, such as adenine, thymine, guanine and cytosine.
Each DNA molecule is made up of two strands of DNA Which are coiled around each other in a form of a spiral ladder.
The bases are present in between the two strands, in a combination of adenine with thymine or cytosine with guanine.
A change in these combinations also leads to mutation. This change in order of these base pairs affect the instructions that are provided to the body to function.
DNA sequencing is referred too as reading of these base pairs. Thus, sequencing of genomes provides a better understanding in causative organism of diseases.
A change or fault in this condition of a DNA causes a genetic condition.
Since this genetic information is passed to their offspring’s through parents they are considered as inherited.
But not everyone from this generation will be affected by this because it also depends upon the passing of traits.
Genetic disorder has the capability to affect any of the genomes which involves many symptoms and causative factors.
Development of Genetic Disorders
Genetic characteristics pass from past generation through future generation between the families.
When parents pass their traits to the children, in some cases they tend to pass their disorders too. Each parent; both father and mother pass half a copy of their genes to their children which is commonly called as allele.
When two form of alleles are passed from parent to the children, the cells in the body of a offspring take the information from only one pair of that allele, which is known as dominant allele, and the other pair of alleles which is least concerned is referred to as recessive allele.
In such cases the person develops a genetic disorder if he gets either one of the dominant alleles or both the recessive allele from the infected parent.
Factors of Genetic Disorders
Genetic disorders are caused by many factors such as single gene inheritance, multifactorial inheritance, chromosomal inheritance and mitochondrial inheritance.
Single Gene Genetic Disorders
Single gene disorder is also known as monogenic disorder.
It is caused due to the mutations in a single gene caused in an individual and they are passed to the upcoming generations due to various factors such as Genomic imprinting and uniparental disomy.
Sometimes these conditions may also cause due to invitro fertilization.
In many cases, the congenital metabolic disorders, which are also known as inborn errors of metabolism are also due to single gene defects.
Single genes disorders also result in autosomal dominant disorders such as Huntington’s disease or Autosomal recessive disorders such as sickle cell disease, cystic fibrosis, phenylketonuria and thalassemia.
It also results in X-linked and Y- linked inherited disorders such as turners’ disorder.
Multifactorial Inheritance
Multifactorial inheritance is caused as a result of combination of both genetic factors and environment influences.
The non-genetic factors that influence the individual is smoking, alcohol, cancer, diabetes, multiple sclerosis and Alzheimer’s disease, etc.
Chromosomal Inheritance
Chromosomal abnormalities are due to the mutation or change in a chromosome of an individual such as having a lesser number of chromosomes than usual, having extra chromosomes or change in structure of any of the chromosomes.
Downs syndrome is one of the examples of chromosomal abnormality.
Mitochondrial Inheritance
This is also due to the single gene inherited disorder, it is also known as maternal inheritance and it occurs in rare cases than usual disorders.
This condition occurs as a result of defect in any of the 13 genes that are encoded by mitochondrial DNA, As the developing embryo gets its mitochondria from the egg cells.
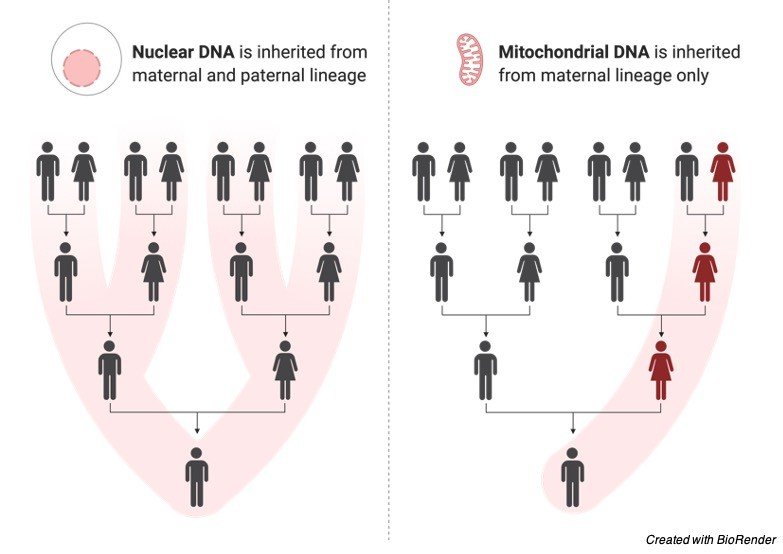
In case the carrying mother is affected this disorder can pass to the offspring.
One of the examples of this type of disorder is Leber’s Hereditary optic neuropathy.
It is also important to know that many mitochondrial disorders are due to defect in the nuclear gene which resembles as such of autosomal recessive inheritance.
Genetic Disorders Diagnosis and Treatment
It is also to be clear that not every genetic disorder leads to death of a progeny. Even though there are no remedies for genetic disorders some can be diagnosed at an early stage.
The genetic disorders such as downs syndrome, and Muscular dystrophy shows no signs until turning into adult.
Even though there is no treatment there is chances of improving the quality of life than degradation such as physiological therapies and management of pain and choosing alternative medications.
Though the treatment of genetic disorders is not yet discovered or it is state of ongoing battle.
However, gene therapy plays an important role in bringing the normal healthy gene into a patient and removing the defective one.
Genetic Disorders Citations
- Genetic disorders. JEMS . 2014 Feb;39(2):64-71.
- Developmental Support for Infants With Genetic Disorders. Pediatrics . 2020 May;145(5):e20190629.
- Genetic Disorders of the Extracellular Matrix. Anat Rec (Hoboken) . 2020 Jun;303(6):1527-1542.
- Genetic disorders of nuclear receptors. J Clin Invest . 2017 Apr 3;127(4):1181-1192.
- Coatopathies: Genetic Disorders of Protein Coats. Annu Rev Cell Dev Biol . 2019 Oct 6;35:131-168.
- Maternal Genetic Disorders in Pregnancy. Obstet Gynecol Clin North Am . 2018 Jun;45(2):249-265.
Share
Similar Post: