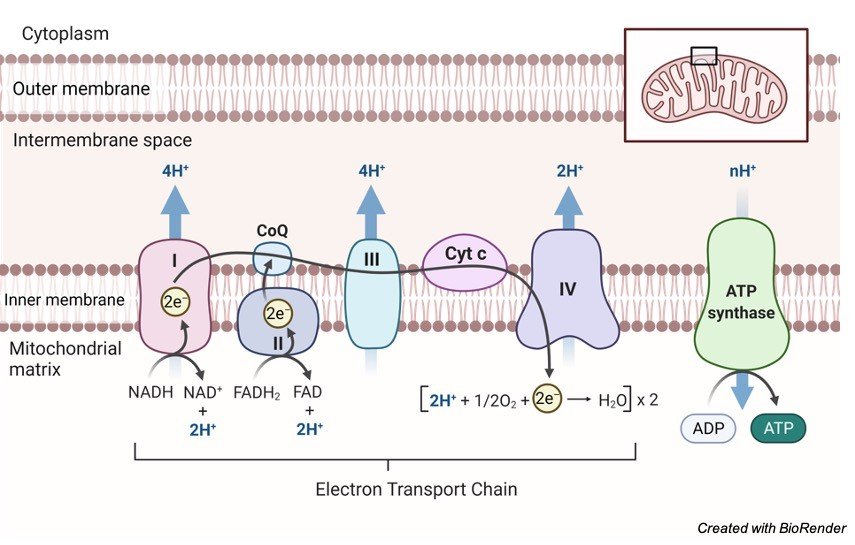
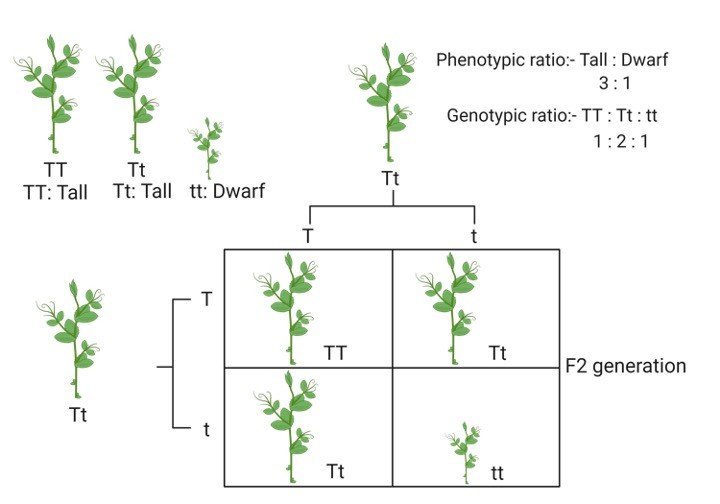
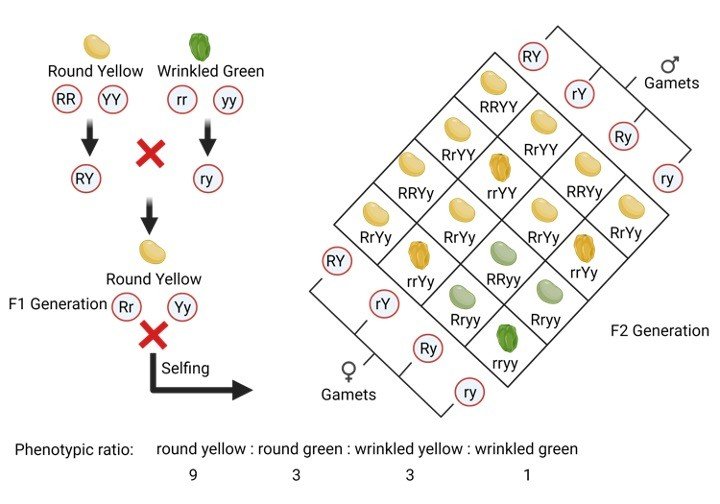
1. Nicotinamide Adenine Dinucleotide (NAD): It is a co-enzyme that carries hydride ion (H-) and thus known as a hydride carrier.
It gains 2 hydrogen atoms from the substrate of Citric acid cycle like isocitrate, malate, B-hydroxy acyl CoA and B-hydroxy butyrate and form NADH molecule.
In the process, one electron releases which reduces NADH to NADH+ while passing its hydrogen to flavoprotein containing FMN and iron sulphur protein (FeS).
2. Flavoproteins (FAD and FMN): In the meanwhile, flavoproteins like FAD and FMN both serves as hydrogen acceptor wherein they tightly bound the hydrogen atom.
Thus, directly preventing its reduced form to react with oxygen.
In general, the types of flavoprotein receiving hydrogen passes them to coenzyme Q.
For example, flavoprotein Fp1 receives 2 hydrogen atoms from reduced NAD+, while flavoprotein Fp2 receiving the same from various substrates like succinate, acyl CoA and choline.
3. Ubiquinone: It is also known as Coenzyme Q that contains quinone ring. Coenzyme Q is the most common ubiquinone that shows similarity with vitamin K.
It is lipid soluble with small molecular size thus responsible for free mobility of the molecule in the inner membrane of mitochondria.
It is the basic carrier of the hydrogen atoms, wherein ubiquinol carries 2 hydrogen atoms while semiquinone carries only one.
These molecules connect flavoprotein with cytochrome b, where flavoprotein carries 2 hydrogen atoms while cytochrome b carry one.
Reduction of coenzyme Q leads to passage of electrons to cytochrome b while releasing 2H+ into the mitochondrial matrix.
The enzymes responsible for the oxidation of ubiquinol involves:
a): Conduction of electrons to cytochrome c is mainly responsible by ubiquinol (coenzyme Q) dehydrogenase along with coenzymes cyt b, FeS protein and cyt c1.
b): Another further transfer from cyt c to oxygen is carried out under the presence of cytochrome oxidase along with cyt a and cyt a3 as coenzymes.
4. Cytochromes are mainly responsible to carry electron from coenzyme Q to oxygen. Thus, exhibits the important role of electron carrier.
They are categorized with the letter a, b and c.
The core molecule for all the cytochromes are haemoproteins however they diverge in their redox potential.
The iron of cytochrome frequently changes their state from oxidation (Fe3+; ferric state) to reduction (Fe2+; Ferrous state) during their normal physiologic action, while that of haemoglobin remains in the reduction state.
Being mobile components, both coenzyme Q and cytochrome c transport reduced equivalent from the other fixed components.
5. Iron Sulfur Clusters(FeS): One additional component found is iron sulfur proteins also known as FeS or non-heme iron.
It is often related with flavoproteins and cytochrome b wherein iron is interchangeably exchange between flavoprotein and cytochrome b resulting in redox reaction.